in Argentina, 2018,
Citation of: Ostroumov SA, Kotelevtsev SV (2011) Toxicology of nanomaterials and environment. Ecologica 18: 3–10 Google Scholar ;
Who cited: top institutions, Ecotoxicology Laboratory, Faculty of Biochemistry and Biological Sciences, FBCB-UNL Ciudad Universitaria, Santa Fe, Argentina; National Council for Scientific and Technical Research (CONICET), Buenos Aires,
**

https://5bio5.blogspot.com/2018/04/citation_10.html
International Journal of Environmental Research;
pp 1–10 | Cite as
Acute Toxicity of Colloidal Silicon Dioxide Nanoparticles on Amphibian Larvae: Emerging Environmental Concern;
Authors,
Authors and affiliations,
Rafael Carlos Lajmanovich, Email author;
Paola Mariela Peltzer,
Candela Soledad Martinuzzi,
Andrés Maximiliano Attademo,
Carlina Leila Colussi,
Agustín Bassó,
Rafael Carlos Lajmanovich,
1
2Email author,
Paola Mariela Peltzer,
1
2
Candela Soledad Martinuzzi,
1
2
Andrés Maximiliano Attademo,
1
2
Carlina Leila Colussi,
1
Agustín Bassó,
1
1.Ecotoxicology Laboratory, Faculty of Biochemistry and Biological Sciences, FBCB-UNL Ciudad Universitaria, Santa Fe, Argentina;
2.National Council for Scientific and Technical Research (CONICET), Buenos Aires, Argentina;
Research paper;
First Online: 06 April 2018;
2 Downloads;
Abstract.
Emerging contaminants derive from pharmaceuticals, pesticides, disinfection by-products, home and care products, and wood preservation and industrial chemicals that contain specific drugs, metals, metal oxides and metalloids as nanoparticles (NPs) in their formulations. Although the use of silicon dioxide (SiO2) NPs in commercial products increases, its impacts on the environment and on animal and human health are largely unknown. Thus, the aim of this study was to evaluate the ecotoxicity of colloidal SiO2-NPs in Rhinella arenarum larvae exposed to 0.001, 0.01, 0.1, and 1 mg/L colloidal SiO2-NPs for 48 h. Biotoxicological endpoints (median lethal concentration-LC50; 95% confidence limits), the no-observed-effect concentration (NOEC), the lowest-observed-effect concentration (LOEC), Toxic Units (TU), oxidative stress enzyme activity (glutathione S-transferase-GST), and genotoxicity (frequency of micronuclei, and other erythrocyte nuclear abnormalities-ENAs) were measured in exposed larvae. Scanning electron microscopy equipped with an energy dispersive X-ray system allowed detecting that SiO2-NPs aggregate on the dorsal skin of SiO2-treated larvae. The 48 h LC50 of colloidal SiO2-NPs was 0.0251 mg/L (0.0163- 0.0338 mg/L). The NOEC and LOEC values after 48 h were 0.001 mg/L and 0.01 mg/L, respectively. According to the hazard classification system for wastewaters discharged into the aquatic environment, the colloidal SiO2-NPs evaluated are Class V, i.e., of very high acute toxicity (TU = 3984.06). At 48 h of exposure to NOEC, GST activity and ENAs frequency were significantly increased (118.75 and 58%, respectively) with respect to controls. The results of the present study indicate that, at low concentration, colloidal SiO2-NPs exerted high toxicity on R. arenarum tadpoles.
Keywords:
Rhinella arenarum, Nanotoxicity, Emerging contaminants, Biomarkers, Personal care products,
Download article PDF
Introduction.
There is increasing widespread concern about the potential impacts of emerging contaminants (ECs) on the environment as well as on wildlife and human health (Kendall et al. 2016). ECs are defined as synthetic or naturally occurring chemicals that have appeared in freshwater ecosystems and potable water during the last decades (Sgroi et al. 2017). Pharmaceuticals, personal care products and endocrine disrupting compounds are among the prime examples of ECs. ECs enter water systems from different sources, such as human excretion (sewage and hospital effluents), clandestine or untreated disposal of animal manure from feedlots, leaching and runoff from agricultural fields that use organic fertilizers, or from industries (Archer et al. 2017). However, monitoring data sets (previously limited by analytical capabilities) and accurate risk assessment and environmental legislation are currently lacking (Lindsey et al. 2001; Klavarioti et al. 2009; Al-Odaini et al. 2013).
Some ECs recently detected in waters are nanoparticles (NPs) (Sauvé and Desrosiers 2014), named as engineered nanomaterials. Nanotechnology has created new type of materials, which have revolutionized the world with a large range of applications (Bhushan 2004). Nanomaterials are diverse types of small-scale materials that have structural elements smaller than 100 nm (nano-sized particles or NPs), in at least one dimension (EPA 2015; Calderón-Jiménez et al. 2017) and have facilitated the development of new cosmetics, pharmaceuticals, personal care products, sunscreen, powdered food, insecticides, and biocidal products for human ectoparasites (e.g., Jones et al. 2008; Gandhi et al. 2016). The use of NPs in cosmetic or personal care products poses significant challenges, because NPs frequently occur at low concentrations and are often incompatible with the analytical instruments that would be required for their identification, quantification and characterization (Contado 2015).
The number of consumer products that have incorporated NPs into their formulations has grown from a total of 54 products identified in 2005 to over 1800 nanomaterial- and NP-containing consumer products in 2014 in 32 countries (Vance et al. 2015). One of the main concerns regarding long-term environmental and human exposures to NPs is the limited information (Sajid et al. 2015). As a consequence, there is a need to evaluate their toxicity (Ray et al. 2009; Ostroumov and Kotelevtsev 2011). In general, the chemical dynamics of NPs is different from that of non-particulate contaminants, so new paradigms will be needed for the NPs present in the water, soil, sediment and biota (Mahdi et al. 2017). Silicon dioxide-based NPs (known as silica) (SiO2-NPs) are one of the main NPs used for personal care products for children and as bio-pesticides for veterinary treatments, human health, and as pesticides in agriculture (Barik et al. 2008, 2012). The products that contain SiO2-NPs in complex matrices such as water and soils (Sferratore et al. 2006) require risk evaluation and characterization in non-target organisms (Sauvé and Desrosiers 2014).
Different investigations have demonstrated that SiO2-NPs in zebrafish (Danio rerio) (embryos and larvae) cause embryonic developmental toxicity by oxidative damage, which results in persistent effects on larval behavior (Duan et al. 2013, Ye et al. 2013). Moreover, Ambrosone et al. (2014) reported that SiO2-NPs treatment of Hydra vulgaris (Cnidaria) leads to the modification of homeostasis and modulation of gene expression. In mice, it has been demonstrated that SiO2-NPs with a diameter of 100 nm induce liver injury (Nishimoria et al. 2009; Hasezaki et al. 2011). Furthermore, insecticides containing NPs such as titanium dioxide NPs (TiO2-NPs) in pediculicidal formulations (Gandhi et al. 2016) act mechanically by obstructing the respiratory openings of the cuticle surfaces of the insects and produce abrasions or block the spiracle, thus leading to biological and behavioral changes, including the reduction of movements, feeding, and death. Likewise, Shrivastava et al. (2007) and Wijnhoven et al. (2009) have suggested that Ag-NPs could accumulate in fish skin and affect cellular modulation and therefore inhibit bacterial growth.
In the present study, larvae of the common South American toad Rhinella arenarum were selected as test organisms. This species has an extended geographical distribution and is frequently present in natural and artificial aquatic ecosystems (e.g., forests, wetlands, agricultural lands, and urban regions) (Bionda et al. 2015). This species is suitable and useful as a laboratory experimental model for monitoring aquatic ecosystems and its sensitivity to some xenobiotics has been proven in many biomarker studies (e.g., Venturino et al. 2003; Lajmanovich et al. 2014). It is important to highlight the ecological role of amphibian tadpoles as a link between terrestrial and aquatic habitats (Altig et al. 2007), in relation to the potential for NPs to be taken up by organisms and be transferred in food webs (Bundschuh et al. 2016).
Some researchers have evaluated the undesired biological effects of ECs (e.g., McConnell and Sparling 2010; Melvin 2016; Peltzer et al. 2017), including some NPs, on amphibian tadpoles (e.g., Hinther et al. 2010; Salvaterra et al. 2013; Nations et al. 2015; Thompson et al. 2017), but little is known about the ecotoxicity of SiO2-NPs on anuran larvae. For these reasons, the aim of this study was to investigate the acute and sublethal effects of colloidal SiO2-NPs using larvae of the common toad R. arenarum as a biological model. The development of such information may allow the assessment and characterization of potential ecological risks following future massive use of NPs as ECs.
Materials and Methods
Test Organisms
Premetamorphic larvae at Gosner stages (GS) 26–30 (Gosner 1960) of R. arenarum (n = 150), with average size (snout-tail tip) 16 ± 0.25 mm and weight 0.045 ± 0.005 g, were collected from the temporary pond called “Lago Parque del Sur” (31°39’53.90″S—60°42′51.20″W, Santa Fe Province, Argentina) in November 2016. In this site, pesticides are never applied because pesticide application is restricted by law because they might have a toxic effect on human and wildlife health (Martinuzzi et al. 2016). Larvae were acclimated in laboratory conditions for 48 h at a 12-h light/dark cycle with dechlorinated tap water (DTW), pH 7.2 ± 0.05, conductivity of 165 ± 12.5 μmhos cm−1, dissolved oxygen concentration of 6.5 ± 1.5 mg L−1, and hardness of 48.5 mg L−1 of CaCO3 at 23 ± 3 °C, and fed on boiled lettuce (Lactuca sativa) at the beginning of the experiment. The bioassays using larvae were approved by the bioethical committee of the Facultad de Bioquímica y Ciencias Biológicas, Universidad del Litoral, Santa Fe, Argentina (Res. CD No.: 388/06), and the experimental protocol was according to the norms of ASIH-American Society of Ichthyologists and Herpetologists (2004) criteria.
NPs and Experimental Design
For short-term (48-h) static toxicity tests, a personal care formulation with a colloidal suspension of SiO2-NPs used to control ectoparasite insects was purchased in a local pharmacy. Sedimentation and re-dispersion were used to obtain only NPs and remove agents and surfactants from the NPs (Anon. 2008). The method consisted in centrifugation at 10,000 rpm for 20 min; and then, to obtain the final concentration of NPs, the pellet was weighed by electronic balance (Ohaus®, ± 0.0001 g) and dissolved in deionized water (Anon 2008; Gandhi et al. 2016). Stock solutions containing colloidal SiO2-NPs were prepared for the toxicity tests. Test concentrations were prepared by diluting the stock solution in DTW. These SiO2-NPs were previously characterized using a Scanning Electron Microscope (SEM) (FEI-QuantaTM200) equipped with an Energy Dispersive X-ray (EDX) system. The same procedure was followed with dehydrated treated larvae. Particles on the images obtained were measured using ImageJ software (available free over the Internet at: http://rsb.info.nih.gov/ij/index.html); the average primary particle diameter was calculated from 20 to 30 particles.
Range-finding toxicity tests consisted in exposing larvae to colloidal SiO2-NPs to estimate the median lethal concentration (LC50), the no-observed-effect concentration (NOEC), and the lowest-observed-effect concentration (LOEC). Ten tadpoles/container were exposed to 0.001, 0.01, 0.1, and 1 mg/L colloidal SiO2-NPs and a control (only DTW) in glass aquaria (13 cm in diameter and 14 cm in height) with 1 L DTW. Both the control and the test solutions were made in triplicate. Treatments were randomly assigned to the experimental containers, as was the order in which the glass containers were sampled. Because of the lack of data on the environmental concentration of NPs deposited on water bodies and uncertainties associated with the fate of these xenobiotics for biomarker assessment, a subsample of tadpoles per control and NOEC-48 h exposures were euthanized in accordance with the ASIH (2004) guidelines and with approval of the animal ethics committee of the Facultad de Bioquímica y Ciencias Biológicas, Universidad del Litoral, Santa Fe, Argentina. The residual water of the experiments was disposed by the Waste Management Program of the same institution.
Antioxidant Enzymes
Each larva was homogenized (on ice) in 0.1% t-octylphenoxypolyethoxy-ethanol (triton X-100) in 25 mM tris (hydroxyl methyl) aminomethane hydrochloride (pH 8.0), using a polytron. Suspensions were centrifuged at 10,000 rpm for 15 min at 4 ± 1 °C and the supernatant (crude extract) was extracted. The Biuret method was used to determine protein concentration in the supernatants (Kingsley 1942). When sample volume was enough, enzyme kinetics assays were carried out in duplicate. Glutathione S-transferase (GST) activity was determined spectrophotometrically using the method described by Habig et al. (1974) and adapted by Habdous et al. (2002) for mammal serum GST activity. The enzyme assay was performed at 340 nm in 100 mM Na–phosphate buffer (pH 6.5) (F.V. = 920 µL), 20 µL of 0.2 mM 1-chloro-2, 4-dinitrobenzene, 50 µL of 5 mM reduced glutathione, and the sample. Enzyme kinetics assays were performed at 25 °C and whole GST activity was expressed as nmol min−1 mg−1 protein using a molar extinction coefficient of 9.6 × 103M−1 cm−1.
Genotoxicity
One smear per larva was prepared on clean slides with blood samples obtained by cardiac puncture, then fixed and stained using the May-Grünwald-Giemsa method (Lajmanovich et al. 2005). It is important to consider that red blood cells in amphibians are nucleated and undergo cell division in the circulation, particularly during the developmental stages. In mature erythrocytes, the frequencies of micronuclei (MN) and other erythrocyte nuclear abnormalities (ENAs) such as binucleated erythrocytes (BER), erythroplastids (EP), kidney-shaped nuclei (KN), lobed nuclei (LN), multi-micronucleated erythrocytes (MMER), and notched nuclei (NN), were recorded according to the procedures of Guilherme et al. (2008). The ENAs value was the sum of BER + EP + KN + LN + MMER + NN (Lajmanovich et al. 2014). Coded and randomized slides were examined blind by a single operator.
Data Analyses
The lethal concentration (LC50) values and their respective 95% confidence limits were calculated using the Trimmed Spearman-Karber method (Hamilton et al. 1977). Mortality data were statistically evaluated using the Dunnett’s test for post hoc comparison of means to determine NOEC and LOEC (U.S.EPA 1989). Taking into account that NPs emitted by wastewater are considered to be widely present in the natural environment (Li et al. 2016), the toxicity value (LC50) was transformed into Toxic Units (TU) according to the following equation: TU = 100/LC50 and classified under the hazard classification system for wastewaters discharged into the aquatic environment (Table 1; Persoone et al. 2003). The data of GST activity were expressed as means ± standard error (SEM). The Mann–Whitney U test was used to compare enzymatic activities between control and SiO2-NPs-treated larvae. Data of MN and other ENAs were analyzed using the binomial proportion test (Margolin et al. 1983). These statistical methods were performed using BioEstat software 5.0 --(Ayres et al. 2008). A value of p < 0.05 was considered significant.
Table 1
Criteria of classification for hazardous substances for wastewaters discharged (Persoone et al. 2003)
TU
Class
Toxicity
< 0.4
Class I
No acute toxicity
< 0.4 < TU < 1
Class II
Slight acute toxicity
1 < TU < 10
Class III
Acute toxicity
10 < TU < 100
Class IV
High acute toxicity
TU > 100
Class V
Very high acute toxicity
Results
SEM analyses of the colloidal SiO2-NPs clearly showed spherical shapes, mostly aggregated, of an average particle size of 102 ± 12 nm (Fig. 1a). EDX spectroscopy confirmed the purity of colloidal SiO2-NPs with high Si contents (Fig. 1b).
Open image in new window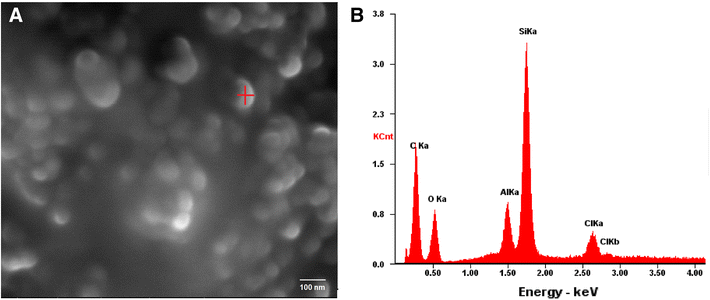
Fig. 1
a Scanning Electron Microscopy (SEM) image of 99% of colloidal silicon dioxide nanoparticles (SiO2-NPs). b SEM–EDX spectra of SiO2-NPs. The vertical axis corresponds to the intensity (counts per second). The highest peak is due to the silice (Si) contents on the sample
Acute Toxicity Tests
No mortality was observed in the controls. The 48 h colloidal SiO2-NPs acute LC50 value (95% CL) calculated based on the Trimmed Spearman-Karber was 0.0251 mg/L (0.0163% - 0.0338 mg/L). The LC50 values were stabilized at 24 h of exposure. The NOEC value was 0.001 mg/L, whereas the LOEC value was 0.01 mg/L. The highest concentration of colloidal SiO2-NPs (1 mg/L) killed all exposed larvae. The value for TU was 3984.06, which, according to the hazard classification system for wastewaters discharged into the aquatic environment, is considered Class V, i.e., of very high acute toxicity (TU > 100; Table 1).
Morphological observations of untreated dehydrated larvae exhibited normal external surface (Fig. 2a). The skin of the larvae treated with 0.01 mg/L colloidal SiO2-NPs (LOEC value) showed several NPs absorbed or adhered, although no intercellular epidermal edemas or focal dermal inflammations were observed (Fig. 2b, c). EDX spectroscopy exhibited the chemical components of these NPs and confirmed the high percentage of Si on the tadpoles skin, which was similar to that of the colloidal SiO2-NPs sample (Fig. 2d).
Open image in new window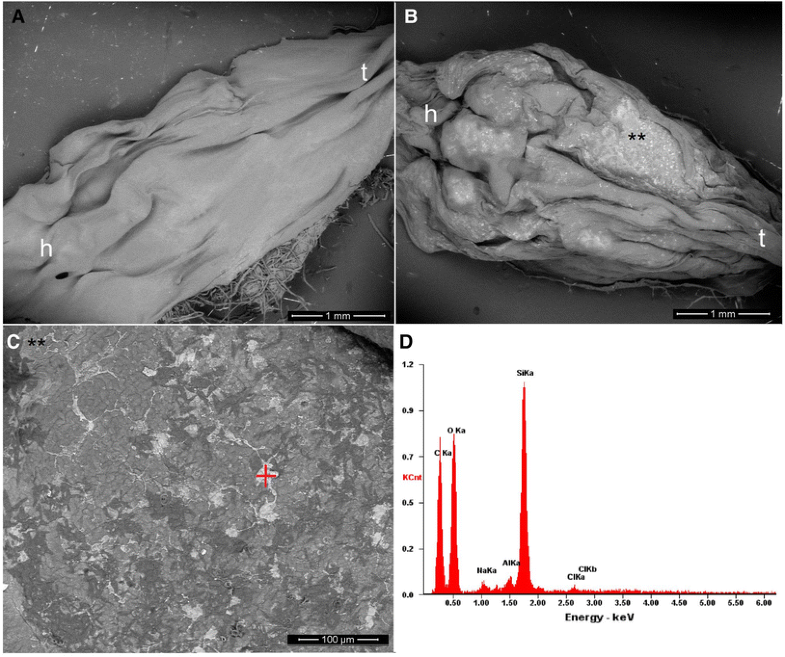
Fig. 2
Scanning electron microscopy (SEM) images of R. arenarum tadpoles (see reference: h head, t tail) exposed to colloidal silicon dioxide nanoparticles (SiO2-NPs). a Control with dechlorinated tap water (DTW). b Tadpole dead at the LOEC value (0.01 mg/L) (**) see detail of SiO2-NPs aggregates adhered to the dorsal skin of R. arenarum larvae. (C) Magnification of area exposed (**) in Fig. 2b. d SEM–EDX spectra for SiO2-NPs aggregates indicated by the red mark in Fig. 2c. The vertical axis corresponds to the intensity (counts per second). The highest peak is due to the silice (Si) contents on the skin sample
GST Activity
The mean value of GST activity in controls was 105.88 ± 12.93 nmol min−1 mg−1 total protein at 48 h. GST activity was highly significantly increased (118.75%) by colloidal SiO2-NPs at NOEC exposure (U = 81.00; p < 0.01) (Fig. 3).
Open image in new window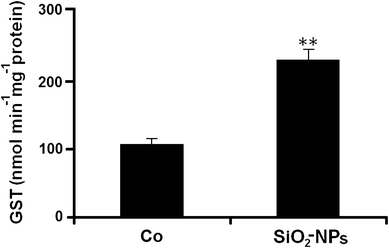
Fig. 3
Glutathione S-transferase (GST) activity in SiO2-NPs-treated R. arenarum larvae at 48 h of exposure. (Co) control. SiO2-NPs: 0.001 mg/L. Bars represent the mean ± SEM, n = 10. Significant difference was **p < 0.01 with respect to the control (Mann–Whitney U test). n = 10
Effect of SiO2-NPs on ENAs
Normal mature erythrocytes of R. arenarum larvae are oblong/oval-shaped with a central nucleus visibly structured and a well-defined boundary, which enabled the recognition of fragments in their cytoplasm (Fig. 4a). The MN quantified were spherical nuclear fragments separated from the nucleus (Fig. 4b). Binucleated, erythroplastid, kidney-shaped, multi-micro nucleated, and notched nuclei were also observed in larvae treated with colloidal SiO2-NPs at the NOEC value (0.001 mg/L) (Fig. 4c–h).
Open image in new window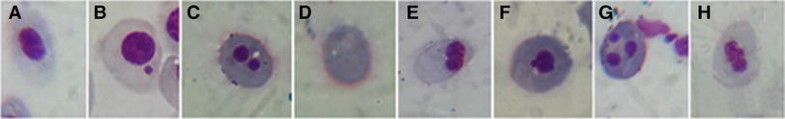
Fig. 4
Detail of red blood cells observed in SiO2-NPs-treated R. arenarum larvae. a normal mature erythrocyte (NE); b micronuclei (MN); c binucleated erythrocyte (BER); d erythroplastid (EP); e kidney-shaped nuclei (KN); g multi-micronucleated erythrocyte (MEER); h notched nuclei (NN). May Grünwald-Giemsa 100X
After 48-h exposure, at the NOEC value of SiO2-NPs (0.001 mg/L), blood of R. arenarum larvae showed a significant increase (58%) in the frequency of ENAs respect to controls (z = − 2.25; p < 0.01) (Fig. 5).
Open image in new window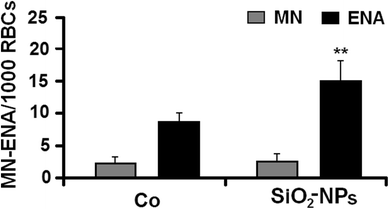
Fig. 5
Induction of micronuclei (MN) and erythrocyte nuclear abnormalities (ENAs) (per 1000 red blood cells; RBCs) in R. arenarum larvae at 48 h of exposure to colloidal silicon dioxide nanoparticles (SiO2-NPs). (Co) control. SiO2-NPs: 0.001 mg/L. Bars represent the mean ± SEM, n = 10. Significant difference was **p < 0.01 with respect to the Co (binomial proportion’s test). n = 10
Discussion
NPs are being increasingly used in imaging, diagnosis, care products, cosmetics, and drug delivery, but their toxicity in aquatic organisms has only recently begun to be investigated. In the present study, very low concentrations (< 1 mg/L) of colloidal SiO2-NPs induced high lethal toxicities in R. arenarum larvae with an LC50 48 h value of 0.0251 mg/L. The TU calculated estimate the risk associated as a result of the discharge of effluents containing these test compounds. As mentioned above, according to the hazard classification system applied for wastewaters discharged into the aquatic environment (Persoone et al. 2003), colloidal SiO2-NPs belong to Class V, which indicates a very high acute toxicity.
It is generally recognized that SiO2-NPs are not toxic (e.g., Ryu et al. 2014; Caltagirone et al. 2015; Diab et al. 2017). However, the increase in the use of NPs in many industry fields has prompted the careful investigation of their toxicity in non-target organisms. Here, we determined different biological effects elicited by SiO2-NPs on R. arenarum larvae. Apparently, as shown in Fig. 2, these NPs were absorbed or adhered onto the tadpole’s skin, possibly by the affinity to lipids and caused the death simply by physical means, similarly to that described for larvicidal and pediculicidal effects by TiO2-NPs (Gandhi et al. 2016). It should be noted that the mode of action of insecticidal compounds for ectoparasite control that contain SiO2-NPs in their formulations is through dehydration of the insect cuticle by physical sorption of lipids, and they are also expected to cause damage in the plasma cell membrane, resulting in cell lysis and death of the organism solely by physical means-asphyxiation mechanism (Tiwari and Behari, 2009). Recent studies on the in vitro toxicity of SiO2-NPs have shown that their toxicity is mediated by adsorption of NPs to extracellular components as serum proteins (Napierska et al. 2010; Zhang et al. 2012). Also, Ambrosone et al. (2014) suggested that, in the case of not-target organisms (e.g., H. vulgaris) in contact with amorphous SiO2-NPs, this interaction first occurs with the external cuticle and induces a progressive morpho-physiological alteration (i.e., changes in hydrostatic pressure) and normal behavior (feeding behavior).
Several studies have reported oxidative stress and pathological changes in aquatic species, specifically in fishes after exposure to TiO2-NPs (e.g., Federici et al. 2007). An increased activity of GST can reveal disorders that could be indicative of redox alterations related to a possible oxidative stress situation (Oruç et al. 2004). After 48 h, R. arenarum larvae treated with colloidal SiO2-NPs at the NOEC value (0.001 mg/L) showed an increase in GST activity in relation to the controls. Similarly, an increase in GST activity induced by SiO2NPs has been reported in various cell lines after 48 of exposure (Munteanu et al. 2010). However, the antioxidant adaptation system to SiO2-NPs is insufficient to prevent the formation of reactive oxygen species (ROS) and thus biomolecules are damaged (Petrache Voicu et al. 2015). SiO2-NPs induce ROS production in R. arenarum larvae and, consequently, a response of antioxidative defences (GST). Similarly, GST activity could be induced to neutralize SiO2-NPs toxicity and could thus be a suitable biomarker for the evaluation of colloidal SiO2-NPs at very low exposure in potentially contaminated aquatic ecosystems.
Some NPs (e.g., TiO2) may generate ROS, which also can lead to DNA damage (Jaeger et al. 2012). For example, Bacchetta et al. (2017) reported genotoxicity and oxidative stress in fish after short-term exposure to silver NPs. MN and other ENAs such as kidney shaped, lobed and segmented nuclear abnormalities, binucleated erythrocytes, erytrhoplastids, kidney-shaped nuclei, lobed nuclei, and others have been used by many authors as suitable indicators for the assessment of genotoxicity of xenobiotics on fishes and amphibians (Ayllon and García-Vazquez 2000; Gravato and Santos 2002; Pacheco and Santos 1997; Lajmanovich et al. 2014), and for natural environment biomonitoring programs (Phan et al. 2007; Attademo et al. 2011). Different frequencies of MN and other ENAs may be caused by specific genotoxic events, which are subjected to different mechanisms of mutagenicity (Bolognesi et al. 2006). The synchronized expressions of ENAs and MN in red blood cells are considered as indicators of cytotoxicity and genetic toxicology, respectively (Grisolia et al. 2009). In the present study, total ENAs of R. arenarum tadpoles exposed to colloidal SiO2-NPs increased significantly. Therefore, this study is the first report investigating the cytotoxic effects of these types of substances by ENAs in amphibians. There is limited evidence concerning whether or not SiO2-NPs are genotoxic and opposed results have been reported (e.g., Wang et al. 2007; Kwon et al. 2014). However, the latest researches confirmed the genotoxic potential of these NPs (e.g., Demir and Castranova, 2016; Åkerlund et al. 2017; Scherzad et al. 2017; de Souza et al. 2018). In this sense, several researches have also pointed out that MN assays are more sensitive and frequently used to confirm the genotoxicity of NPs (Kisin et al. 2007; Landsiedel et al. 2009). The molecular mechanisms (genotoxicity, cytotoxicity) of SiO2-NPs are not quite clear and further investigations are needed.
Conclusions
Many reports about the toxicity of SiO2-NPs either mention the effects of pure ingredients or are carried out in vitro or in cell cultures. However, the present results showed the biotoxicity effects of SiO2-NPs contained in a biocidal commercial product on a non-target aquatic vertebrate. The LC50 here recorded for SiO2-NPs-treated R. arenarum larvae indicates that high toxicities were induced by very low concentrations of this xenobiotic. The present results indicate that these NPs have high acute biotoxicity and are thus potentially deleterious to aquatic ecosystems. Also, colloidal SiO2-NPs at NOEC value can induce signs of cytotoxicity. However, further studies are necessary to elucidate the role of the possible synergy of the surfactants, additives, and dispersing agents with SiO2-NPs within a commercial product that can produce high lethal toxicity, oxidative stress, and genotoxicity in amphibian larvae.
A combination of ecotoxicological studies on non-target organisms and a multi-scale monitoring program, fate and risk assessment tools with respect to NPs as emerging contaminants is required as a base for sustainable water resource management and overall protection of ecological communities in the aquatic environment.
Notes
Acknowledgements
The scanning electronic microscope micrographs and the energy dispersive X-ray spectroscopy analysis were conducted at the Instituto de Física de Rosario (IFIR) of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Rosario, Argentina. We thank Dr. Martina Avalos for her help with micrographs, spectroscopy, and related discussions and Dr. Maria Victoria Eusevi for English Editing Service. This study was supported in part by CONICET, National Agency for Promotion of Science and Technology, and Course of Action for Research and Science Promotion (CAI + D-UNL), Argentina.
References
Åkerlund E, Cappellini F, Di Bucchianico S, Islam S, Skoglund S, Derr R, Odnevall Wallinder I, Hendriks G, Karlsson HL (2017) Genotoxic and mutagenic properties of Ni and NiO nanoparticles investigated by comet assay, γ-H2AX staining, Hprt mutation assay and ToxTracker reporter cell lines. Environ Mol Mutagen. https://doi.org/10.1002/em.22163 (epub ahead of print) Google Scholar
Al-Odaini NA, Zakaria MP, Yaziz MI, Surif S, Abdulghani M (2013) The occurrence of human pharmaceuticals in wastewater effluents and surface water of Langat River and its tributaries, Malaysia. Int J Environ Anal Chem 93(3):245–264. https://doi.org/10.1080/03067319.2011.592949 CrossRefGoogle Scholar
Altig R, Whiles MR, Taylor CL (2007) What do tadpoles really eat? Assessing the trophic status of an understudied and imperiled group of consumers in freshwater habitats. Freshw Biol 52:386–395. https://doi.org/10.1111/j.1365-2427.2006.01694.x CrossRefGoogle Scholar
Ambrosone A, Scotto di Vettimo MR, Malvindi MA, Roopin M, Levy O, Marchesano V, Pompa PP, Tortiglione C, Tino A (2014) Impact of amorphous SiO2 nanoparticles on a living organism: morphological, behavioral, and molecular biology implications. Int J Environ Anal Chem. https://doi.org/10.3389/fbioe.2014.00037 Google Scholar
Anon. (2008) Working with microspheres, Bangs Laboratories, Inc., 9025 Technology Dr., Fishers, IN 46038-2886, TechNote 201, pp 20Google Scholar
Archer E, Petrie B, Kasprzyk-Hordern B, Wolfaardt GM (2017) The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 174:437–446. https://doi.org/10.1016/j.chemosphere.2017.01.101 CrossRefGoogle Scholar
ASIH-American Society of Ichthyologists and Herpetologists (2004) Guidelines for use of live amphibians and reptiles in field and laboratory research. Herpetological Animal Care and Use Committee (HACC), Washington DCGoogle Scholar
Attademo AM, Cabagna Zenklusen M, Lajmanovich RC, Peltzer PM, Junges C, Bassó A (2011) B-Esterase activities and blood cell morphology in the Frog Leptodactylus chaquensis (Amphibia: Leptodactylidae) on rice agroecosystems from Santa Fe Province (Argentina). Ecotoxicology 20:274–282. https://doi.org/10.1007/s10646-010-0579-8 CrossRefGoogle Scholar
Ayllon F, García-Vazquez E (2000) Induction of micronuclei and other nuclear abnormalities in European minnow Phoxinus phoxinus and mollie Poecilia latipinna: na assessment of the fish micronucleus test. Mutat Res 467:177–186. https://doi.org/10.1016/S1383-5718(00)00033-4 CrossRefGoogle Scholar
Ayres M Jr, Ayres D, Santos A (2008) BioEstat, Versão5.0. Sociedade Civil Mamirauá, MCT-CNPq, Belém, BrazilGoogle Scholar
Bacchetta C, Ale A, Simoniello MF, Gervasio S, Davico C, Rossi AS, Desimone MF, Poletta G, López G, Monserrat JM, Cazenave J (2017) Genotoxicity and oxidative stress in fish after a short-term exposure to silver nanoparticles. Ecol Indic 76:230–239. https://doi.org/10.1016/j.ecolind.2017.01.018 CrossRefGoogle Scholar
Barik TK, Sahu B, Swain V (2008) Nanosilica-from medicine to pest control. Parasitol Res 103(2):253–258. https://doi.org/10.1007/s00436-008-0975-7 CrossRefGoogle Scholar
Barik TK, Kamaraju R, Gowswami A (2012) Silica nanoparticle: a potential new insecticide for mosquito vector control. Parasitol Res 111(3):1075–1083. https://doi.org/10.1007/s00436-012-2934-6 CrossRefGoogle Scholar
Bhushan B (2004) Springer handbook of nanotechnology. Springer, New YorkCrossRefGoogle Scholar
Bionda CL, Kost S, Salas NE, Lajmanovich RC, Sinsch U, Martino AL (2015) Age structure, growth and longevity in the common toad, Rhinella arenarum, from Argentina. Acta Herpetol 10:55–62. https://doi.org/10.13128/Acta_Herpetol-15142 Google Scholar
Bolognesi C, Perrone E, Roggieri P, Pampanin DM, Sciutto A (2006) Assessment of micronuclei induction in peripheral erythrocytes of fish exposed to xenobiotics under controlled conditions. Aquat Toxicol 78:93–98. https://doi.org/10.1016/j.aquatox.2006.02.015 CrossRefGoogle Scholar
Bundschuh M, Seitz F, Rosenfeldt RR, Schulz R (2016) Effects of nanoparticles in fresh waters: risks, mechanisms and interactions. Freshw Biol 61:2185–2196. https://doi.org/10.1111/fwb.12701 CrossRefGoogle Scholar
Calderón-Jiménez B, Johnson M, Montoro Bustos A, Murphy C, Winchester M, Vega Baudrit J (2017) Silver nanoparticles: technological advances, societal impacts, and metrological challenges. Front Chem. https://doi.org/10.3389/fchem.2017.00006 Google Scholar
Caltagirone C, Bettoschi A, Garau A, Montis R (2015) Silica-based nanoparticles: a versatile ool for the development of efficient imaging agents. Chem Soc Rev 44:4645–4671. https://doi.org/10.1039/c4cs00270a CrossRefGoogle Scholar
Contado C (2015) Nanomaterials in consumer products: a challenging analytical problem. Front Chem. https://doi.org/10.3389/fchem.2015.00048 Google Scholar
de Souza TAJ, Rocha TL, Franchi LP (2018) Detection of DNA damage induced by cerium dioxide nanoparticles: from models to molecular mechanism activated. Adv Exp Med Biol 1048:215–226. https://doi.org/10.1007/978-3-319-72041-8_13 CrossRefGoogle Scholar
Demir E, Castranova V (2016) Genotoxic effects of synthetic amorphous silica nanoparticles in the mouse lymphoma assay. Toxicol Rep 3:807–815. https://doi.org/10.1016/j.toxrep.2016.10.006 CrossRefGoogle Scholar
Diab R, Canilho N, Pavel IA, Haffner FB, Girardon M, Pasc A (2017) Silica-based systems for oral delivery of drugs, macromolecules and cells. Adv Colloid Interface Sci 249:346–362. https://doi.org/10.1016/j.cis.2017.04.005 CrossRefGoogle Scholar
Duan J, Yu Y, Shi H, Tian L, Guo C, Huang P, Zhou X, Peng S, Sun Z (2013) Toxic effects of silica nanoparticles on zebrafish embryos and larvae. PLoS ONE 8(9):e74606. https://doi.org/10.1371/journal.pone.0074606 CrossRefGoogle Scholar
Environmental Protection Agency EPA (2015) Chemical substances when manufactured or processed as nanoscale materials: TSCA reporting and recordkeeping requirements Fed. Regist. 80, 18330. http://www.regulations.gov. Accessed 12 July 2016
Federici G, Shaw BJ, Handy RD (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol 84:415–430. https://doi.org/10.1016/j.aquatox.2007.07.009 CrossRefGoogle Scholar
Gandhi PR, Jayaseelan C, Vimalkumar E, Mary RR (2016) Larvicidal and pediculicidal activity of synthesized TiO2 nanoparticles using Vitex negundo leaf extract against blood feeding parasites. Asia Pac Entomol 19:1089–1094. https://doi.org/10.1016/j.aspen.2016.10.001 CrossRefGoogle Scholar
Gosner KL (1960) A simplified table for staging anuran embryos and larvae, with notes on identification. Herpetologica 16:183–190Google Scholar
Gravato C, Santos MA (2002) B-Naphthoflavone liver EROD and erythrocytic nuclear abnormality induction in juvenile Dicentrarchus labrax L. Ecotoxicol Environ Saf 52:69–74. https://doi.org/10.1006/eesa.2002.2151 CrossRefGoogle Scholar
Grisolia CK, Rivero CLG, Starling FLRM, Silva ICR, Barbosa AC, Dorea JG (2009) Profile of micronucleus frequencies and DNA damage in different species of fish in a eutrophic tropical lake. Genet Mol Biol 32:138–143. https://doi.org/10.1590/S1415-47572009005000009 CrossRefGoogle Scholar
Guilherme S, Válega M, Pereira ME, Santos MA, Pacheco M (2008) Erythrocytic nuclear abnormalities in wild and caged fish (Liza aurata) along an environmental Mercury contamination gradient. Ecotoxicol Environ Saf 70:411–421. https://doi.org/10.1016/j.ecoenv.2007.08.016 CrossRefGoogle Scholar
Habdous M, Vincent-Viry M, Visvikis S, Siest G (2002) Rapid spectrophotometric method for serum glutathione S-transferases activity. Clin Chim Acta 326:131–142. https://doi.org/10.1016/S0009-8981(02)00329-7 CrossRefGoogle Scholar
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139Google Scholar
Hamilton MA, Russo RC, Thurston RV (1977) Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol 11:714–719. https://doi.org/10.1021/es60130a004 CrossRefGoogle Scholar
Hasezaki T, Isoda K, Kondoh M, Tsutsumi Y, Yagi K (2011) Hepatotoxicity of silica nanoparticles with a diameter of 100 nm. Pharmazie 66(9):698–703. https://doi.org/10.1691/ph.2011.1516 Google Scholar
Hinther A, Vawda S, Skirrow RC, Veldhoen N, Collins P, Cullen JT, van Aggelen G, Helbing CC (2010) Nanometals induce stress and alter thyroid hormone action in amphibia at or below North American water quality guidelines. Environ Sci Technol 44:8314–8321. https://doi.org/10.1021/es101902n CrossRefGoogle Scholar
Jaeger A, Weiss DG, Jonas L, Kriehuber R (2012) Oxidative stress-induced cytotoxic and genotoxic effects of nano-sized titanium dioxide particles in human HaCaT keratinocytes. Toxicology 296:27–36. https://doi.org/10.1016/j.tox.2012.02.016 CrossRefGoogle Scholar
Jones N, Ray B, Ranjit KT, Manna AC (2008) Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 279:71–76. https://doi.org/10.1111/j.1574-6968.2007.01012.x CrossRefGoogle Scholar
Kendall RJ, Lacher TE, Cobb GC, Cox SB (2016) Wildlife toxicology: emerging contaminant and biodiversity issues. CRC Press, New YorkGoogle Scholar
Kingsley GR (1942) The direct biuret method for the determination of serum proteins as applied to photoelectric and visual calorimetry. J Lab Clin Med 27:840–845Google Scholar
Kisin ER, Murray AR, Keane MJ, Shi XC, Schwegler-Berry D, Gorelik O, Arepalli S, Castranova V, Wallace WE, Kagan VE, Shvedova AA (2007) Single-walled carbon nanotubes: geno- and cytotoxic effects in lung fibroblast V79 cells. J Toxicol Environ Health A 70(24):2071–2079. https://doi.org/10.1080/15287390701601251 CrossRefGoogle Scholar
Klavarioti M, Mantzavinos D, Kassinos D (2009) Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ Int 35(2):402–417. https://doi.org/10.1016/j.envint.2008.07.009 CrossRefGoogle Scholar
Kwon JY, Kim HL, Lee JY, Ju YH, Kim JS, Kang SH, Kim YR, Lee JK, Jeong J, Kim MK, Maeng EH, Seo YR (2014) Undetactable levels of genotoxicity of SiO2 nanoparticles in in vitro and in vivo tests. Int J Nanomed 9(Suppl 2):173–1781. https://doi.org/10.2147/ijn.s57933 Google Scholar
Lajmanovich RC, Cabagna M, Peltzer PM, Stringhini GA, Attademo AM (2005) Micronucleus induction in erythrocytes of the Hyla pulchella tadpoles (Amphibia: Hylidae) exposed to insecticide endosulfan. Mutat Res 10:67–72. https://doi.org/10.1016/j.mrgentox.2005.08.001 CrossRefGoogle Scholar
Lajmanovich RC, Cabagna Zenklusen M, Attademo AM, Junges CM, Peltzer PM, Bassó A, Lorenzatti E (2014) Induction of micronuclei and nuclear abnormalities in common toad tadpoles (Rhinella arenarum) treated with Liberty® and glufosinate-ammonium. Mutat Res Genet Toxicol Environ Mutagen 15(769):7–12. https://doi.org/10.1016/j.mrgentox.2014.04.009 CrossRefGoogle Scholar
Landsiedel R, Kapp MD, Schulz M, Wiench K, Oesch F (2009) Genotoxicity investigations on nanomaterials: methods, preparation and characterization of test material, potential artifacts and limitations—many questions, some answers. Mutat Res 681(2–3):241–258. https://doi.org/10.1016/j.mrrev.2008.10.002 CrossRefGoogle Scholar
Li L, Stoiber M, Wimmer A, Xu Z, Lindenblatt C, Helmreich B, Schuster M (2016) To what extent can full-scale wastewater treatment plant effluent influence the occurrence of silver-based nanoparticles in surface waters? Environ Sci Technol 50:6327–6333. https://doi.org/10.1021/acs.est.6b00694 CrossRefGoogle Scholar
Lindsey ME, Meyer M, Thurman EM (2001) Analysis of trace levels of sulfonamide and tetracycline antimicrobials in groundwater and surface water using solid-phase extraction and liquid chromatography/mass spectrometry. Anal Chem 73(19):4640–4646. https://doi.org/10.1021/ac010514w CrossRefGoogle Scholar
Mahdi KNM, Commelin M, Peters RJB, Baartman JEM, Ritsema C, Geissen V (2017) Transport of silver nanoparticles by runoff and erosion—a flume experiment. Sci Total Environ 601–602:1418–1426. https://doi.org/10.1016/j.scitotenv.2017.06.020 CrossRefGoogle Scholar
Margolin BH, Collings BJ, Mason JM (1983) Statistical analysis and sample- size determinations for mutagenicity experiments with binomial responses. Environ Mutagen 5:705–716. https://doi.org/10.1002/em.2860050509 CrossRefGoogle Scholar
Martinuzzi C, Peltzer PM, Attademo AM, Junges CM, Lajmanovich RC (2016) Albinism in larvae of the Chacoan frog Leptodactylus chaquensis (Anura, Leptodactylidae) from an urban lake from Argentina. Cuadernos de Herpetología 30(2):69–73Google Scholar
McConnell L, Sparling DW (2010) Emerging contaminants and their potential effects on amphibians and reptiles. In: Sparling DW, Linder G, Bishop C, Krest S (eds) Ecotoxicology of amphibians and reptiles, 2nd edn. SETAC Press, Pensacola, pp 498–513Google Scholar
Melvin SD (2016) Oxidative stress, energy storage, and swimming performance of Limnodynastes peronii tadpoles exposed to a sub-lethal pharmaceutical mixture throughout development. Chemosphere 150:790–797. https://doi.org/10.1016/j.chemosphere.2015.09.034 CrossRefGoogle Scholar
Munteanu MC, Radu M, Hermenean A, Sima C, Dinu D, Costache M, Grigoriu C, Dinischiotu A (2010) Antioxidative response induced by SiO2 nanoparticles in MRC-5 cell line. Rom Biotech Lett 5(1):5000–5007Google Scholar
Napierska D, Thomassen LCJ, Lison D, Martens JA, Hoet PH (2010) The nanosilica hazard: another variable entity. Fibre Toxicol, Part. https://doi.org/10.1186/1743-8977-7-39 Google Scholar
Nations S, Long M, Wages M, Maul JD, Theodorakis CW, Cobb GP (2015) Subchronic and chronic developmental effects of copper oxide (CuO) nanoparticles on Xenopus laevis. Chemosphere 135:166–174. https://doi.org/10.1016/j.chemosphere.2015.03.078 CrossRefGoogle Scholar
Nishimoria H, Kondoha M, Isodaa K, Tsunodabc S, Tsutsumibcd Y, Yagia Y (2009) Silica nanoparticles as hepatotoxicants. Eur J Pharm Biopharm 72(3):496–501. https://doi.org/10.1016/j.ejpb.2009.02.005 CrossRefGoogle Scholar
Oruç EO, Sevgiler Y, Uner N (2004) Tissue-specific oxidative stress responses in fish exposed to 2,4-D and azinphosmethyl. Comp Biochem Physiol C 137(1):43–51. https://doi.org/10.1016/j.cca.2003.11.006 CrossRefGoogle Scholar
Ostroumov SA, Kotelevtsev SV (2011) Toxicology of nanomaterials and environment. Ecologica 18: 3–10 Google Scholar
Pacheco M, Santos MA (1997) Induction of EROD activity and genotoxic effects by polycyclic aromatic hydrocarbons and resin acids on the juvenile eell (Anguilla anguilla L.). Ecotoxicol Environ Saf 38:252–259. https://doi.org/10.1006/eesa.1997.1585 CrossRefGoogle Scholar
Peltzer PM, Lajmanovich RC, Attademo AM, Junges CM, Teglia CM, Martinuzzi C, Curi L, Culzoni MJ, Goicoechea HC (2017) Ecotoxicity of veterinary enrofloxacin and ciprofloxacin antibiotics on anuran amphibian larvae. Environ Toxicol Pharmacol 51:114–123. https://doi.org/10.1016/j.etap.2017.01.021 CrossRefGoogle Scholar
Persoone G, Marsalek B, Blinova I, Törökne A, Zarina D, Manusadzianas L, Nalecz-Jawecki G, Tofan L, Stepanova N, Tothova L, Kolar B (2003) A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ Toxicol 18(6):395–402. https://doi.org/10.1002/tox.10141 CrossRefGoogle Scholar
Petrache Voicu SN, Dinu D, Sima C, Hermenean A, Ardelean A, Codrici E, Stan MS, Zărnescu O, Dinischiotu A (2015) Silica nanoparticles induce oxidative stress and autophagy but not apoptosis in the MRC-5 cell line. Int J Mol Sci 16(12):29398–29416. https://doi.org/10.3390/ijms161226171 CrossRefGoogle Scholar
Phan VN, Gomes V, Passos MJACR, Ussami KA, Campos DYF, Rocha AJS, Pereira BA (2007) Biomonitoring of the genotoxic potential (micronucleus and erythrocyte nuclear abnormalities assay) of the Admiralty Bay water surrounding the Brazilian Antarctic Research Station Comandante Ferraz King George Island. Polar Biol 30(2):209–217. https://doi.org/10.4322/apa.2015.020 CrossRefGoogle Scholar
Ray PC, Yu H, Fu PP (2009) Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Health C 27(1):1–35. https://doi.org/10.1080/10590500802708267 CrossRefGoogle Scholar
Ryu HJ, Seong NW, So BJ, Seo H, Kim JH, Hong JS, Park MK, Kim MS, Kim YR, Cho KB, Seo MY, Kim MK, Maeng EH, Son SW (2014) Evaluation of silica nanoparticle toxicity after topical exposure for 90 days. Int J Nanomed 9(Suppl 2):127–136. https://doi.org/10.2147/IJN.S57929 Google Scholar
Sajid M, Ilyas M, Basheer C, Tariq M, Daud M, Baig N, Shehzad F (2015) Impact of nanoparticles on human and environment: review of toxicity factors, exposures, control strategies, and future prospects. Environ Sci Pollut Res Int 22(6):4122–4143. https://doi.org/10.1007/s11356-014-3994-1 CrossRefGoogle Scholar
Salvaterra T, Alves MG, Domingues I, Pereira R, Rasteiro MG, Carvalho RA, Soares AMVM, Lopes I (2013) Biochemical and metabolic effects of a short-term exposure to nanoparticles of titanium silicate in tadpoles of Pelophylax perezi (Seoane). Aquat Toxicol 128–129:190–192. https://doi.org/10.1016/j.aquatox.2012.12.014 CrossRefGoogle Scholar
Sauvé S, Desrosiers M (2014) A review of what is an emerging contaminant. Chem Cent J 8:15. https://doi.org/10.1186/1752-153X-8-15 CrossRefGoogle Scholar
Scherzad A, Meyer T, Kleinsasser N, Hackenberg S (2017) Molecular mechanisms of zinc oxide nanoparticle-induced genotoxicity. Materials (Basel) 10:1427. https://doi.org/10.3390/ma10121427 CrossRefGoogle Scholar
Sferratore A, Garnier J, Billen G, Conley DI, Pinault S (2006) Diffuse and point sources of silica in the Seine River watershed. Environ Sci Technol 40(21):6630–6635. https://doi.org/10.1021/es060710q CrossRefGoogle Scholar
Sgroi M, Roccaro P, Korshin GV, Vagliasindi FG (2017) Monitoring the behavior of emerging contaminants in wastewater-impacted rivers based on the use of fluorescence excitation emission matrixes (EEM). Environ Sci Technol 51(8):4306–4316. https://doi.org/10.1021/acs.est.6b05785 CrossRefGoogle Scholar
Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 18(22):1–9. https://doi.org/10.1088/0957-4484/18/22/225103 CrossRefGoogle Scholar
Thompson LB, Carfagno GLF, Andresen K, Sitton AJ, Bury T, Lee LL, Lerner KT, Fong PP (2017) Differential uptake of gold nanoparticles by 2 species of tadpole, the wood frog (Lithobates sylvaticus) and the bullfrog (Lithobates catesbeianus). Environ Toxicol Chem. https://doi.org/10.1002/etc.3909 (epub ahead of print) Google Scholar
Tiwari DK, Behari J (2009) Biocidal nature of treatment of Ag-nanoparticle and ultrasonic irradiation in Escherichia coli dh5. Adv Biol Res 3(3–4):89–95Google Scholar
U.S.EPA (U.S. Environmental Protection Agency) (1989) Short-term methods for estimating the chronic toxicity of effluents and receiving waters to fresh water organisms, Report EPA/600/4-89/001. Environmental Protection Agency, CincinnatiGoogle Scholar
Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF Jr, Rejeski D (2015) Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol 6:1769–1780. https://doi.org/10.3762/bjnano.6.181 CrossRefGoogle Scholar
Venturino A, Rosenbaum E, Caballero De Castro A, Anguiano O, Gauna L, Fonovich de Schroeder T, Pechen de D’Angelo AM (2003) Biomarkers of effect in toads and frogs. Biomarkers 8(3–4):167–186. https://doi.org/10.1080/1354700031000120116 CrossRefGoogle Scholar
Wang JJ, Sanderson BJS, Wang H (2007) Cytotoxicity and genotoxicity of ultrafine crystalline SiO2 particulate in cultured human lymphoblastoid cells. Environ Mol Mutagen 48:151–157. https://doi.org/10.1002/em.20287 CrossRefGoogle Scholar
Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, Roszek B, Bisschops J, Gosens I, van de Meent D, Dekkers S, de Jong WH, van Zijverden M, Sips AJAM, Geertsma RE (2009) Nano-silver: a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 3(2):109–138. https://doi.org/10.1080/17435390902725914 CrossRefGoogle Scholar
Ye RF, Yu XW, Yang SY, Yuan JL, Yang X (2013) Effects of silica dioxide nanoparticles on the embryonic development of zebrafish. Integr Ferroelectr 147:166–174. https://doi.org/10.1080/10584587.2013.792625 CrossRefGoogle Scholar
Zhang HY, Dunphy DR, Jiang XM, Meng H, Sun BB, Tarn D, Xue M, Wang X, Lin S, Ji Z, Li R, Garcia FL, Yang J, Kirk ML, Xia T, Zink JI, Nel A, Brinker CJ (2012) Processing pathway dependence of amorphous silica nanoparticle toxicity: colloidal vs pyrolytic. J Am Chem Soc 134(38):15790–15804. https://doi.org/10.1021/ja304907c CrossRefGoogle Scholar
Copyright information
© University of Tehran 2018
About this article
CrossMark
Cite this article as: Lajmanovich, R.C., Peltzer, P.M., Martinuzzi, C.S. et al. Int J Environ Res (2018). https://doi.org/10.1007/s41742-018-0089-8
DOI https://doi.org/10.1007/s41742-018-0089-8
Publisher Name Springer International Publishing
Print ISSN 1735-6865
Online ISSN 2008-2304
About this journal
Reprints and Permissions
Personalised recommendations
Download PDF
Actions
Download PDF
How to cite?
.RIS Papers Reference Manager RefWorks Zotero
.ENW EndNote
.BIB BibTeX JabRef Mendeley Share article
Table of contents
Article
Abstract
Introduction
Materials and Methods
Results
Discussion
Conclusions
Notes
References
Copyright information
About this article
Advertisement
**
Citation of: Ostroumov SA, Kotelevtsev SV (2011) Toxicology of nanomaterials and environment. Ecologica 18: 3–10 Google Scholar ;
Who cited: top institutions, Ecotoxicology Laboratory, Faculty of Biochemistry and Biological Sciences, FBCB-UNL Ciudad Universitaria, Santa Fe, Argentina; National Council for Scientific and Technical Research (CONICET), Buenos Aires,
**
https://5bio5.blogspot.com/2018/04/citation_10.html
International Journal of Environmental Research;
pp 1–10 | Cite as
Acute Toxicity of Colloidal Silicon Dioxide Nanoparticles on Amphibian Larvae: Emerging Environmental Concern;
Authors,
Authors and affiliations,
Rafael Carlos Lajmanovich, Email author;
Paola Mariela Peltzer,
Candela Soledad Martinuzzi,
Andrés Maximiliano Attademo,
Carlina Leila Colussi,
Agustín Bassó,
Rafael Carlos Lajmanovich,
1
2Email author,
Paola Mariela Peltzer,
1
2
Candela Soledad Martinuzzi,
1
2
Andrés Maximiliano Attademo,
1
2
Carlina Leila Colussi,
1
Agustín Bassó,
1
1.Ecotoxicology Laboratory, Faculty of Biochemistry and Biological Sciences, FBCB-UNL Ciudad Universitaria, Santa Fe, Argentina;
2.National Council for Scientific and Technical Research (CONICET), Buenos Aires, Argentina;
Research paper;
First Online: 06 April 2018;
2 Downloads;
Abstract.
Emerging contaminants derive from pharmaceuticals, pesticides, disinfection by-products, home and care products, and wood preservation and industrial chemicals that contain specific drugs, metals, metal oxides and metalloids as nanoparticles (NPs) in their formulations. Although the use of silicon dioxide (SiO2) NPs in commercial products increases, its impacts on the environment and on animal and human health are largely unknown. Thus, the aim of this study was to evaluate the ecotoxicity of colloidal SiO2-NPs in Rhinella arenarum larvae exposed to 0.001, 0.01, 0.1, and 1 mg/L colloidal SiO2-NPs for 48 h. Biotoxicological endpoints (median lethal concentration-LC50; 95% confidence limits), the no-observed-effect concentration (NOEC), the lowest-observed-effect concentration (LOEC), Toxic Units (TU), oxidative stress enzyme activity (glutathione S-transferase-GST), and genotoxicity (frequency of micronuclei, and other erythrocyte nuclear abnormalities-ENAs) were measured in exposed larvae. Scanning electron microscopy equipped with an energy dispersive X-ray system allowed detecting that SiO2-NPs aggregate on the dorsal skin of SiO2-treated larvae. The 48 h LC50 of colloidal SiO2-NPs was 0.0251 mg/L (0.0163- 0.0338 mg/L). The NOEC and LOEC values after 48 h were 0.001 mg/L and 0.01 mg/L, respectively. According to the hazard classification system for wastewaters discharged into the aquatic environment, the colloidal SiO2-NPs evaluated are Class V, i.e., of very high acute toxicity (TU = 3984.06). At 48 h of exposure to NOEC, GST activity and ENAs frequency were significantly increased (118.75 and 58%, respectively) with respect to controls. The results of the present study indicate that, at low concentration, colloidal SiO2-NPs exerted high toxicity on R. arenarum tadpoles.
Keywords:
Rhinella arenarum, Nanotoxicity, Emerging contaminants, Biomarkers, Personal care products,
Download article PDF
Introduction.
There is increasing widespread concern about the potential impacts of emerging contaminants (ECs) on the environment as well as on wildlife and human health (Kendall et al. 2016). ECs are defined as synthetic or naturally occurring chemicals that have appeared in freshwater ecosystems and potable water during the last decades (Sgroi et al. 2017). Pharmaceuticals, personal care products and endocrine disrupting compounds are among the prime examples of ECs. ECs enter water systems from different sources, such as human excretion (sewage and hospital effluents), clandestine or untreated disposal of animal manure from feedlots, leaching and runoff from agricultural fields that use organic fertilizers, or from industries (Archer et al. 2017). However, monitoring data sets (previously limited by analytical capabilities) and accurate risk assessment and environmental legislation are currently lacking (Lindsey et al. 2001; Klavarioti et al. 2009; Al-Odaini et al. 2013).
Some ECs recently detected in waters are nanoparticles (NPs) (Sauvé and Desrosiers 2014), named as engineered nanomaterials. Nanotechnology has created new type of materials, which have revolutionized the world with a large range of applications (Bhushan 2004). Nanomaterials are diverse types of small-scale materials that have structural elements smaller than 100 nm (nano-sized particles or NPs), in at least one dimension (EPA 2015; Calderón-Jiménez et al. 2017) and have facilitated the development of new cosmetics, pharmaceuticals, personal care products, sunscreen, powdered food, insecticides, and biocidal products for human ectoparasites (e.g., Jones et al. 2008; Gandhi et al. 2016). The use of NPs in cosmetic or personal care products poses significant challenges, because NPs frequently occur at low concentrations and are often incompatible with the analytical instruments that would be required for their identification, quantification and characterization (Contado 2015).
The number of consumer products that have incorporated NPs into their formulations has grown from a total of 54 products identified in 2005 to over 1800 nanomaterial- and NP-containing consumer products in 2014 in 32 countries (Vance et al. 2015). One of the main concerns regarding long-term environmental and human exposures to NPs is the limited information (Sajid et al. 2015). As a consequence, there is a need to evaluate their toxicity (Ray et al. 2009; Ostroumov and Kotelevtsev 2011). In general, the chemical dynamics of NPs is different from that of non-particulate contaminants, so new paradigms will be needed for the NPs present in the water, soil, sediment and biota (Mahdi et al. 2017). Silicon dioxide-based NPs (known as silica) (SiO2-NPs) are one of the main NPs used for personal care products for children and as bio-pesticides for veterinary treatments, human health, and as pesticides in agriculture (Barik et al. 2008, 2012). The products that contain SiO2-NPs in complex matrices such as water and soils (Sferratore et al. 2006) require risk evaluation and characterization in non-target organisms (Sauvé and Desrosiers 2014).
Different investigations have demonstrated that SiO2-NPs in zebrafish (Danio rerio) (embryos and larvae) cause embryonic developmental toxicity by oxidative damage, which results in persistent effects on larval behavior (Duan et al. 2013, Ye et al. 2013). Moreover, Ambrosone et al. (2014) reported that SiO2-NPs treatment of Hydra vulgaris (Cnidaria) leads to the modification of homeostasis and modulation of gene expression. In mice, it has been demonstrated that SiO2-NPs with a diameter of 100 nm induce liver injury (Nishimoria et al. 2009; Hasezaki et al. 2011). Furthermore, insecticides containing NPs such as titanium dioxide NPs (TiO2-NPs) in pediculicidal formulations (Gandhi et al. 2016) act mechanically by obstructing the respiratory openings of the cuticle surfaces of the insects and produce abrasions or block the spiracle, thus leading to biological and behavioral changes, including the reduction of movements, feeding, and death. Likewise, Shrivastava et al. (2007) and Wijnhoven et al. (2009) have suggested that Ag-NPs could accumulate in fish skin and affect cellular modulation and therefore inhibit bacterial growth.
In the present study, larvae of the common South American toad Rhinella arenarum were selected as test organisms. This species has an extended geographical distribution and is frequently present in natural and artificial aquatic ecosystems (e.g., forests, wetlands, agricultural lands, and urban regions) (Bionda et al. 2015). This species is suitable and useful as a laboratory experimental model for monitoring aquatic ecosystems and its sensitivity to some xenobiotics has been proven in many biomarker studies (e.g., Venturino et al. 2003; Lajmanovich et al. 2014). It is important to highlight the ecological role of amphibian tadpoles as a link between terrestrial and aquatic habitats (Altig et al. 2007), in relation to the potential for NPs to be taken up by organisms and be transferred in food webs (Bundschuh et al. 2016).
Some researchers have evaluated the undesired biological effects of ECs (e.g., McConnell and Sparling 2010; Melvin 2016; Peltzer et al. 2017), including some NPs, on amphibian tadpoles (e.g., Hinther et al. 2010; Salvaterra et al. 2013; Nations et al. 2015; Thompson et al. 2017), but little is known about the ecotoxicity of SiO2-NPs on anuran larvae. For these reasons, the aim of this study was to investigate the acute and sublethal effects of colloidal SiO2-NPs using larvae of the common toad R. arenarum as a biological model. The development of such information may allow the assessment and characterization of potential ecological risks following future massive use of NPs as ECs.
Materials and Methods
Test Organisms
Premetamorphic larvae at Gosner stages (GS) 26–30 (Gosner 1960) of R. arenarum (n = 150), with average size (snout-tail tip) 16 ± 0.25 mm and weight 0.045 ± 0.005 g, were collected from the temporary pond called “Lago Parque del Sur” (31°39’53.90″S—60°42′51.20″W, Santa Fe Province, Argentina) in November 2016. In this site, pesticides are never applied because pesticide application is restricted by law because they might have a toxic effect on human and wildlife health (Martinuzzi et al. 2016). Larvae were acclimated in laboratory conditions for 48 h at a 12-h light/dark cycle with dechlorinated tap water (DTW), pH 7.2 ± 0.05, conductivity of 165 ± 12.5 μmhos cm−1, dissolved oxygen concentration of 6.5 ± 1.5 mg L−1, and hardness of 48.5 mg L−1 of CaCO3 at 23 ± 3 °C, and fed on boiled lettuce (Lactuca sativa) at the beginning of the experiment. The bioassays using larvae were approved by the bioethical committee of the Facultad de Bioquímica y Ciencias Biológicas, Universidad del Litoral, Santa Fe, Argentina (Res. CD No.: 388/06), and the experimental protocol was according to the norms of ASIH-American Society of Ichthyologists and Herpetologists (2004) criteria.
NPs and Experimental Design
For short-term (48-h) static toxicity tests, a personal care formulation with a colloidal suspension of SiO2-NPs used to control ectoparasite insects was purchased in a local pharmacy. Sedimentation and re-dispersion were used to obtain only NPs and remove agents and surfactants from the NPs (Anon. 2008). The method consisted in centrifugation at 10,000 rpm for 20 min; and then, to obtain the final concentration of NPs, the pellet was weighed by electronic balance (Ohaus®, ± 0.0001 g) and dissolved in deionized water (Anon 2008; Gandhi et al. 2016). Stock solutions containing colloidal SiO2-NPs were prepared for the toxicity tests. Test concentrations were prepared by diluting the stock solution in DTW. These SiO2-NPs were previously characterized using a Scanning Electron Microscope (SEM) (FEI-QuantaTM200) equipped with an Energy Dispersive X-ray (EDX) system. The same procedure was followed with dehydrated treated larvae. Particles on the images obtained were measured using ImageJ software (available free over the Internet at: http://rsb.info.nih.gov/ij/index.html); the average primary particle diameter was calculated from 20 to 30 particles.
Range-finding toxicity tests consisted in exposing larvae to colloidal SiO2-NPs to estimate the median lethal concentration (LC50), the no-observed-effect concentration (NOEC), and the lowest-observed-effect concentration (LOEC). Ten tadpoles/container were exposed to 0.001, 0.01, 0.1, and 1 mg/L colloidal SiO2-NPs and a control (only DTW) in glass aquaria (13 cm in diameter and 14 cm in height) with 1 L DTW. Both the control and the test solutions were made in triplicate. Treatments were randomly assigned to the experimental containers, as was the order in which the glass containers were sampled. Because of the lack of data on the environmental concentration of NPs deposited on water bodies and uncertainties associated with the fate of these xenobiotics for biomarker assessment, a subsample of tadpoles per control and NOEC-48 h exposures were euthanized in accordance with the ASIH (2004) guidelines and with approval of the animal ethics committee of the Facultad de Bioquímica y Ciencias Biológicas, Universidad del Litoral, Santa Fe, Argentina. The residual water of the experiments was disposed by the Waste Management Program of the same institution.
Antioxidant Enzymes
Each larva was homogenized (on ice) in 0.1% t-octylphenoxypolyethoxy-ethanol (triton X-100) in 25 mM tris (hydroxyl methyl) aminomethane hydrochloride (pH 8.0), using a polytron. Suspensions were centrifuged at 10,000 rpm for 15 min at 4 ± 1 °C and the supernatant (crude extract) was extracted. The Biuret method was used to determine protein concentration in the supernatants (Kingsley 1942). When sample volume was enough, enzyme kinetics assays were carried out in duplicate. Glutathione S-transferase (GST) activity was determined spectrophotometrically using the method described by Habig et al. (1974) and adapted by Habdous et al. (2002) for mammal serum GST activity. The enzyme assay was performed at 340 nm in 100 mM Na–phosphate buffer (pH 6.5) (F.V. = 920 µL), 20 µL of 0.2 mM 1-chloro-2, 4-dinitrobenzene, 50 µL of 5 mM reduced glutathione, and the sample. Enzyme kinetics assays were performed at 25 °C and whole GST activity was expressed as nmol min−1 mg−1 protein using a molar extinction coefficient of 9.6 × 103M−1 cm−1.
Genotoxicity
One smear per larva was prepared on clean slides with blood samples obtained by cardiac puncture, then fixed and stained using the May-Grünwald-Giemsa method (Lajmanovich et al. 2005). It is important to consider that red blood cells in amphibians are nucleated and undergo cell division in the circulation, particularly during the developmental stages. In mature erythrocytes, the frequencies of micronuclei (MN) and other erythrocyte nuclear abnormalities (ENAs) such as binucleated erythrocytes (BER), erythroplastids (EP), kidney-shaped nuclei (KN), lobed nuclei (LN), multi-micronucleated erythrocytes (MMER), and notched nuclei (NN), were recorded according to the procedures of Guilherme et al. (2008). The ENAs value was the sum of BER + EP + KN + LN + MMER + NN (Lajmanovich et al. 2014). Coded and randomized slides were examined blind by a single operator.
Data Analyses
The lethal concentration (LC50) values and their respective 95% confidence limits were calculated using the Trimmed Spearman-Karber method (Hamilton et al. 1977). Mortality data were statistically evaluated using the Dunnett’s test for post hoc comparison of means to determine NOEC and LOEC (U.S.EPA 1989). Taking into account that NPs emitted by wastewater are considered to be widely present in the natural environment (Li et al. 2016), the toxicity value (LC50) was transformed into Toxic Units (TU) according to the following equation: TU = 100/LC50 and classified under the hazard classification system for wastewaters discharged into the aquatic environment (Table 1; Persoone et al. 2003). The data of GST activity were expressed as means ± standard error (SEM). The Mann–Whitney U test was used to compare enzymatic activities between control and SiO2-NPs-treated larvae. Data of MN and other ENAs were analyzed using the binomial proportion test (Margolin et al. 1983). These statistical methods were performed using BioEstat software 5.0 --(Ayres et al. 2008). A value of p < 0.05 was considered significant.
Table 1
Criteria of classification for hazardous substances for wastewaters discharged (Persoone et al. 2003)
TU
Class
Toxicity
< 0.4
Class I
No acute toxicity
< 0.4 < TU < 1
Class II
Slight acute toxicity
1 < TU < 10
Class III
Acute toxicity
10 < TU < 100
Class IV
High acute toxicity
TU > 100
Class V
Very high acute toxicity
Results
SEM analyses of the colloidal SiO2-NPs clearly showed spherical shapes, mostly aggregated, of an average particle size of 102 ± 12 nm (Fig. 1a). EDX spectroscopy confirmed the purity of colloidal SiO2-NPs with high Si contents (Fig. 1b).
Open image in new window

Fig. 1
a Scanning Electron Microscopy (SEM) image of 99% of colloidal silicon dioxide nanoparticles (SiO2-NPs). b SEM–EDX spectra of SiO2-NPs. The vertical axis corresponds to the intensity (counts per second). The highest peak is due to the silice (Si) contents on the sample
Acute Toxicity Tests
No mortality was observed in the controls. The 48 h colloidal SiO2-NPs acute LC50 value (95% CL) calculated based on the Trimmed Spearman-Karber was 0.0251 mg/L (0.0163% - 0.0338 mg/L). The LC50 values were stabilized at 24 h of exposure. The NOEC value was 0.001 mg/L, whereas the LOEC value was 0.01 mg/L. The highest concentration of colloidal SiO2-NPs (1 mg/L) killed all exposed larvae. The value for TU was 3984.06, which, according to the hazard classification system for wastewaters discharged into the aquatic environment, is considered Class V, i.e., of very high acute toxicity (TU > 100; Table 1).
Morphological observations of untreated dehydrated larvae exhibited normal external surface (Fig. 2a). The skin of the larvae treated with 0.01 mg/L colloidal SiO2-NPs (LOEC value) showed several NPs absorbed or adhered, although no intercellular epidermal edemas or focal dermal inflammations were observed (Fig. 2b, c). EDX spectroscopy exhibited the chemical components of these NPs and confirmed the high percentage of Si on the tadpoles skin, which was similar to that of the colloidal SiO2-NPs sample (Fig. 2d).
Open image in new window

Fig. 2
Scanning electron microscopy (SEM) images of R. arenarum tadpoles (see reference: h head, t tail) exposed to colloidal silicon dioxide nanoparticles (SiO2-NPs). a Control with dechlorinated tap water (DTW). b Tadpole dead at the LOEC value (0.01 mg/L) (**) see detail of SiO2-NPs aggregates adhered to the dorsal skin of R. arenarum larvae. (C) Magnification of area exposed (**) in Fig. 2b. d SEM–EDX spectra for SiO2-NPs aggregates indicated by the red mark in Fig. 2c. The vertical axis corresponds to the intensity (counts per second). The highest peak is due to the silice (Si) contents on the skin sample
GST Activity
The mean value of GST activity in controls was 105.88 ± 12.93 nmol min−1 mg−1 total protein at 48 h. GST activity was highly significantly increased (118.75%) by colloidal SiO2-NPs at NOEC exposure (U = 81.00; p < 0.01) (Fig. 3).
Open image in new window

Fig. 3
Glutathione S-transferase (GST) activity in SiO2-NPs-treated R. arenarum larvae at 48 h of exposure. (Co) control. SiO2-NPs: 0.001 mg/L. Bars represent the mean ± SEM, n = 10. Significant difference was **p < 0.01 with respect to the control (Mann–Whitney U test). n = 10
Effect of SiO2-NPs on ENAs
Normal mature erythrocytes of R. arenarum larvae are oblong/oval-shaped with a central nucleus visibly structured and a well-defined boundary, which enabled the recognition of fragments in their cytoplasm (Fig. 4a). The MN quantified were spherical nuclear fragments separated from the nucleus (Fig. 4b). Binucleated, erythroplastid, kidney-shaped, multi-micro nucleated, and notched nuclei were also observed in larvae treated with colloidal SiO2-NPs at the NOEC value (0.001 mg/L) (Fig. 4c–h).
Open image in new window

Fig. 4
Detail of red blood cells observed in SiO2-NPs-treated R. arenarum larvae. a normal mature erythrocyte (NE); b micronuclei (MN); c binucleated erythrocyte (BER); d erythroplastid (EP); e kidney-shaped nuclei (KN); g multi-micronucleated erythrocyte (MEER); h notched nuclei (NN). May Grünwald-Giemsa 100X
After 48-h exposure, at the NOEC value of SiO2-NPs (0.001 mg/L), blood of R. arenarum larvae showed a significant increase (58%) in the frequency of ENAs respect to controls (z = − 2.25; p < 0.01) (Fig. 5).
Open image in new window

Fig. 5
Induction of micronuclei (MN) and erythrocyte nuclear abnormalities (ENAs) (per 1000 red blood cells; RBCs) in R. arenarum larvae at 48 h of exposure to colloidal silicon dioxide nanoparticles (SiO2-NPs). (Co) control. SiO2-NPs: 0.001 mg/L. Bars represent the mean ± SEM, n = 10. Significant difference was **p < 0.01 with respect to the Co (binomial proportion’s test). n = 10
Discussion
NPs are being increasingly used in imaging, diagnosis, care products, cosmetics, and drug delivery, but their toxicity in aquatic organisms has only recently begun to be investigated. In the present study, very low concentrations (< 1 mg/L) of colloidal SiO2-NPs induced high lethal toxicities in R. arenarum larvae with an LC50 48 h value of 0.0251 mg/L. The TU calculated estimate the risk associated as a result of the discharge of effluents containing these test compounds. As mentioned above, according to the hazard classification system applied for wastewaters discharged into the aquatic environment (Persoone et al. 2003), colloidal SiO2-NPs belong to Class V, which indicates a very high acute toxicity.
It is generally recognized that SiO2-NPs are not toxic (e.g., Ryu et al. 2014; Caltagirone et al. 2015; Diab et al. 2017). However, the increase in the use of NPs in many industry fields has prompted the careful investigation of their toxicity in non-target organisms. Here, we determined different biological effects elicited by SiO2-NPs on R. arenarum larvae. Apparently, as shown in Fig. 2, these NPs were absorbed or adhered onto the tadpole’s skin, possibly by the affinity to lipids and caused the death simply by physical means, similarly to that described for larvicidal and pediculicidal effects by TiO2-NPs (Gandhi et al. 2016). It should be noted that the mode of action of insecticidal compounds for ectoparasite control that contain SiO2-NPs in their formulations is through dehydration of the insect cuticle by physical sorption of lipids, and they are also expected to cause damage in the plasma cell membrane, resulting in cell lysis and death of the organism solely by physical means-asphyxiation mechanism (Tiwari and Behari, 2009). Recent studies on the in vitro toxicity of SiO2-NPs have shown that their toxicity is mediated by adsorption of NPs to extracellular components as serum proteins (Napierska et al. 2010; Zhang et al. 2012). Also, Ambrosone et al. (2014) suggested that, in the case of not-target organisms (e.g., H. vulgaris) in contact with amorphous SiO2-NPs, this interaction first occurs with the external cuticle and induces a progressive morpho-physiological alteration (i.e., changes in hydrostatic pressure) and normal behavior (feeding behavior).
Several studies have reported oxidative stress and pathological changes in aquatic species, specifically in fishes after exposure to TiO2-NPs (e.g., Federici et al. 2007). An increased activity of GST can reveal disorders that could be indicative of redox alterations related to a possible oxidative stress situation (Oruç et al. 2004). After 48 h, R. arenarum larvae treated with colloidal SiO2-NPs at the NOEC value (0.001 mg/L) showed an increase in GST activity in relation to the controls. Similarly, an increase in GST activity induced by SiO2NPs has been reported in various cell lines after 48 of exposure (Munteanu et al. 2010). However, the antioxidant adaptation system to SiO2-NPs is insufficient to prevent the formation of reactive oxygen species (ROS) and thus biomolecules are damaged (Petrache Voicu et al. 2015). SiO2-NPs induce ROS production in R. arenarum larvae and, consequently, a response of antioxidative defences (GST). Similarly, GST activity could be induced to neutralize SiO2-NPs toxicity and could thus be a suitable biomarker for the evaluation of colloidal SiO2-NPs at very low exposure in potentially contaminated aquatic ecosystems.
Some NPs (e.g., TiO2) may generate ROS, which also can lead to DNA damage (Jaeger et al. 2012). For example, Bacchetta et al. (2017) reported genotoxicity and oxidative stress in fish after short-term exposure to silver NPs. MN and other ENAs such as kidney shaped, lobed and segmented nuclear abnormalities, binucleated erythrocytes, erytrhoplastids, kidney-shaped nuclei, lobed nuclei, and others have been used by many authors as suitable indicators for the assessment of genotoxicity of xenobiotics on fishes and amphibians (Ayllon and García-Vazquez 2000; Gravato and Santos 2002; Pacheco and Santos 1997; Lajmanovich et al. 2014), and for natural environment biomonitoring programs (Phan et al. 2007; Attademo et al. 2011). Different frequencies of MN and other ENAs may be caused by specific genotoxic events, which are subjected to different mechanisms of mutagenicity (Bolognesi et al. 2006). The synchronized expressions of ENAs and MN in red blood cells are considered as indicators of cytotoxicity and genetic toxicology, respectively (Grisolia et al. 2009). In the present study, total ENAs of R. arenarum tadpoles exposed to colloidal SiO2-NPs increased significantly. Therefore, this study is the first report investigating the cytotoxic effects of these types of substances by ENAs in amphibians. There is limited evidence concerning whether or not SiO2-NPs are genotoxic and opposed results have been reported (e.g., Wang et al. 2007; Kwon et al. 2014). However, the latest researches confirmed the genotoxic potential of these NPs (e.g., Demir and Castranova, 2016; Åkerlund et al. 2017; Scherzad et al. 2017; de Souza et al. 2018). In this sense, several researches have also pointed out that MN assays are more sensitive and frequently used to confirm the genotoxicity of NPs (Kisin et al. 2007; Landsiedel et al. 2009). The molecular mechanisms (genotoxicity, cytotoxicity) of SiO2-NPs are not quite clear and further investigations are needed.
Conclusions
Many reports about the toxicity of SiO2-NPs either mention the effects of pure ingredients or are carried out in vitro or in cell cultures. However, the present results showed the biotoxicity effects of SiO2-NPs contained in a biocidal commercial product on a non-target aquatic vertebrate. The LC50 here recorded for SiO2-NPs-treated R. arenarum larvae indicates that high toxicities were induced by very low concentrations of this xenobiotic. The present results indicate that these NPs have high acute biotoxicity and are thus potentially deleterious to aquatic ecosystems. Also, colloidal SiO2-NPs at NOEC value can induce signs of cytotoxicity. However, further studies are necessary to elucidate the role of the possible synergy of the surfactants, additives, and dispersing agents with SiO2-NPs within a commercial product that can produce high lethal toxicity, oxidative stress, and genotoxicity in amphibian larvae.
A combination of ecotoxicological studies on non-target organisms and a multi-scale monitoring program, fate and risk assessment tools with respect to NPs as emerging contaminants is required as a base for sustainable water resource management and overall protection of ecological communities in the aquatic environment.
Notes
Acknowledgements
The scanning electronic microscope micrographs and the energy dispersive X-ray spectroscopy analysis were conducted at the Instituto de Física de Rosario (IFIR) of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Rosario, Argentina. We thank Dr. Martina Avalos for her help with micrographs, spectroscopy, and related discussions and Dr. Maria Victoria Eusevi for English Editing Service. This study was supported in part by CONICET, National Agency for Promotion of Science and Technology, and Course of Action for Research and Science Promotion (CAI + D-UNL), Argentina.
References
Åkerlund E, Cappellini F, Di Bucchianico S, Islam S, Skoglund S, Derr R, Odnevall Wallinder I, Hendriks G, Karlsson HL (2017) Genotoxic and mutagenic properties of Ni and NiO nanoparticles investigated by comet assay, γ-H2AX staining, Hprt mutation assay and ToxTracker reporter cell lines. Environ Mol Mutagen. https://doi.org/10.1002/em.22163 (epub ahead of print) Google Scholar
Al-Odaini NA, Zakaria MP, Yaziz MI, Surif S, Abdulghani M (2013) The occurrence of human pharmaceuticals in wastewater effluents and surface water of Langat River and its tributaries, Malaysia. Int J Environ Anal Chem 93(3):245–264. https://doi.org/10.1080/03067319.2011.592949 CrossRefGoogle Scholar
Altig R, Whiles MR, Taylor CL (2007) What do tadpoles really eat? Assessing the trophic status of an understudied and imperiled group of consumers in freshwater habitats. Freshw Biol 52:386–395. https://doi.org/10.1111/j.1365-2427.2006.01694.x CrossRefGoogle Scholar
Ambrosone A, Scotto di Vettimo MR, Malvindi MA, Roopin M, Levy O, Marchesano V, Pompa PP, Tortiglione C, Tino A (2014) Impact of amorphous SiO2 nanoparticles on a living organism: morphological, behavioral, and molecular biology implications. Int J Environ Anal Chem. https://doi.org/10.3389/fbioe.2014.00037 Google Scholar
Anon. (2008) Working with microspheres, Bangs Laboratories, Inc., 9025 Technology Dr., Fishers, IN 46038-2886, TechNote 201, pp 20Google Scholar
Archer E, Petrie B, Kasprzyk-Hordern B, Wolfaardt GM (2017) The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 174:437–446. https://doi.org/10.1016/j.chemosphere.2017.01.101 CrossRefGoogle Scholar
ASIH-American Society of Ichthyologists and Herpetologists (2004) Guidelines for use of live amphibians and reptiles in field and laboratory research. Herpetological Animal Care and Use Committee (HACC), Washington DCGoogle Scholar
Attademo AM, Cabagna Zenklusen M, Lajmanovich RC, Peltzer PM, Junges C, Bassó A (2011) B-Esterase activities and blood cell morphology in the Frog Leptodactylus chaquensis (Amphibia: Leptodactylidae) on rice agroecosystems from Santa Fe Province (Argentina). Ecotoxicology 20:274–282. https://doi.org/10.1007/s10646-010-0579-8 CrossRefGoogle Scholar
Ayllon F, García-Vazquez E (2000) Induction of micronuclei and other nuclear abnormalities in European minnow Phoxinus phoxinus and mollie Poecilia latipinna: na assessment of the fish micronucleus test. Mutat Res 467:177–186. https://doi.org/10.1016/S1383-5718(00)00033-4 CrossRefGoogle Scholar
Ayres M Jr, Ayres D, Santos A (2008) BioEstat, Versão5.0. Sociedade Civil Mamirauá, MCT-CNPq, Belém, BrazilGoogle Scholar
Bacchetta C, Ale A, Simoniello MF, Gervasio S, Davico C, Rossi AS, Desimone MF, Poletta G, López G, Monserrat JM, Cazenave J (2017) Genotoxicity and oxidative stress in fish after a short-term exposure to silver nanoparticles. Ecol Indic 76:230–239. https://doi.org/10.1016/j.ecolind.2017.01.018 CrossRefGoogle Scholar
Barik TK, Sahu B, Swain V (2008) Nanosilica-from medicine to pest control. Parasitol Res 103(2):253–258. https://doi.org/10.1007/s00436-008-0975-7 CrossRefGoogle Scholar
Barik TK, Kamaraju R, Gowswami A (2012) Silica nanoparticle: a potential new insecticide for mosquito vector control. Parasitol Res 111(3):1075–1083. https://doi.org/10.1007/s00436-012-2934-6 CrossRefGoogle Scholar
Bhushan B (2004) Springer handbook of nanotechnology. Springer, New YorkCrossRefGoogle Scholar
Bionda CL, Kost S, Salas NE, Lajmanovich RC, Sinsch U, Martino AL (2015) Age structure, growth and longevity in the common toad, Rhinella arenarum, from Argentina. Acta Herpetol 10:55–62. https://doi.org/10.13128/Acta_Herpetol-15142 Google Scholar
Bolognesi C, Perrone E, Roggieri P, Pampanin DM, Sciutto A (2006) Assessment of micronuclei induction in peripheral erythrocytes of fish exposed to xenobiotics under controlled conditions. Aquat Toxicol 78:93–98. https://doi.org/10.1016/j.aquatox.2006.02.015 CrossRefGoogle Scholar
Bundschuh M, Seitz F, Rosenfeldt RR, Schulz R (2016) Effects of nanoparticles in fresh waters: risks, mechanisms and interactions. Freshw Biol 61:2185–2196. https://doi.org/10.1111/fwb.12701 CrossRefGoogle Scholar
Calderón-Jiménez B, Johnson M, Montoro Bustos A, Murphy C, Winchester M, Vega Baudrit J (2017) Silver nanoparticles: technological advances, societal impacts, and metrological challenges. Front Chem. https://doi.org/10.3389/fchem.2017.00006 Google Scholar
Caltagirone C, Bettoschi A, Garau A, Montis R (2015) Silica-based nanoparticles: a versatile ool for the development of efficient imaging agents. Chem Soc Rev 44:4645–4671. https://doi.org/10.1039/c4cs00270a CrossRefGoogle Scholar
Contado C (2015) Nanomaterials in consumer products: a challenging analytical problem. Front Chem. https://doi.org/10.3389/fchem.2015.00048 Google Scholar
de Souza TAJ, Rocha TL, Franchi LP (2018) Detection of DNA damage induced by cerium dioxide nanoparticles: from models to molecular mechanism activated. Adv Exp Med Biol 1048:215–226. https://doi.org/10.1007/978-3-319-72041-8_13 CrossRefGoogle Scholar
Demir E, Castranova V (2016) Genotoxic effects of synthetic amorphous silica nanoparticles in the mouse lymphoma assay. Toxicol Rep 3:807–815. https://doi.org/10.1016/j.toxrep.2016.10.006 CrossRefGoogle Scholar
Diab R, Canilho N, Pavel IA, Haffner FB, Girardon M, Pasc A (2017) Silica-based systems for oral delivery of drugs, macromolecules and cells. Adv Colloid Interface Sci 249:346–362. https://doi.org/10.1016/j.cis.2017.04.005 CrossRefGoogle Scholar
Duan J, Yu Y, Shi H, Tian L, Guo C, Huang P, Zhou X, Peng S, Sun Z (2013) Toxic effects of silica nanoparticles on zebrafish embryos and larvae. PLoS ONE 8(9):e74606. https://doi.org/10.1371/journal.pone.0074606 CrossRefGoogle Scholar
Environmental Protection Agency EPA (2015) Chemical substances when manufactured or processed as nanoscale materials: TSCA reporting and recordkeeping requirements Fed. Regist. 80, 18330. http://www.regulations.gov. Accessed 12 July 2016
Federici G, Shaw BJ, Handy RD (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol 84:415–430. https://doi.org/10.1016/j.aquatox.2007.07.009 CrossRefGoogle Scholar
Gandhi PR, Jayaseelan C, Vimalkumar E, Mary RR (2016) Larvicidal and pediculicidal activity of synthesized TiO2 nanoparticles using Vitex negundo leaf extract against blood feeding parasites. Asia Pac Entomol 19:1089–1094. https://doi.org/10.1016/j.aspen.2016.10.001 CrossRefGoogle Scholar
Gosner KL (1960) A simplified table for staging anuran embryos and larvae, with notes on identification. Herpetologica 16:183–190Google Scholar
Gravato C, Santos MA (2002) B-Naphthoflavone liver EROD and erythrocytic nuclear abnormality induction in juvenile Dicentrarchus labrax L. Ecotoxicol Environ Saf 52:69–74. https://doi.org/10.1006/eesa.2002.2151 CrossRefGoogle Scholar
Grisolia CK, Rivero CLG, Starling FLRM, Silva ICR, Barbosa AC, Dorea JG (2009) Profile of micronucleus frequencies and DNA damage in different species of fish in a eutrophic tropical lake. Genet Mol Biol 32:138–143. https://doi.org/10.1590/S1415-47572009005000009 CrossRefGoogle Scholar
Guilherme S, Válega M, Pereira ME, Santos MA, Pacheco M (2008) Erythrocytic nuclear abnormalities in wild and caged fish (Liza aurata) along an environmental Mercury contamination gradient. Ecotoxicol Environ Saf 70:411–421. https://doi.org/10.1016/j.ecoenv.2007.08.016 CrossRefGoogle Scholar
Habdous M, Vincent-Viry M, Visvikis S, Siest G (2002) Rapid spectrophotometric method for serum glutathione S-transferases activity. Clin Chim Acta 326:131–142. https://doi.org/10.1016/S0009-8981(02)00329-7 CrossRefGoogle Scholar
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139Google Scholar
Hamilton MA, Russo RC, Thurston RV (1977) Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol 11:714–719. https://doi.org/10.1021/es60130a004 CrossRefGoogle Scholar
Hasezaki T, Isoda K, Kondoh M, Tsutsumi Y, Yagi K (2011) Hepatotoxicity of silica nanoparticles with a diameter of 100 nm. Pharmazie 66(9):698–703. https://doi.org/10.1691/ph.2011.1516 Google Scholar
Hinther A, Vawda S, Skirrow RC, Veldhoen N, Collins P, Cullen JT, van Aggelen G, Helbing CC (2010) Nanometals induce stress and alter thyroid hormone action in amphibia at or below North American water quality guidelines. Environ Sci Technol 44:8314–8321. https://doi.org/10.1021/es101902n CrossRefGoogle Scholar
Jaeger A, Weiss DG, Jonas L, Kriehuber R (2012) Oxidative stress-induced cytotoxic and genotoxic effects of nano-sized titanium dioxide particles in human HaCaT keratinocytes. Toxicology 296:27–36. https://doi.org/10.1016/j.tox.2012.02.016 CrossRefGoogle Scholar
Jones N, Ray B, Ranjit KT, Manna AC (2008) Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 279:71–76. https://doi.org/10.1111/j.1574-6968.2007.01012.x CrossRefGoogle Scholar
Kendall RJ, Lacher TE, Cobb GC, Cox SB (2016) Wildlife toxicology: emerging contaminant and biodiversity issues. CRC Press, New YorkGoogle Scholar
Kingsley GR (1942) The direct biuret method for the determination of serum proteins as applied to photoelectric and visual calorimetry. J Lab Clin Med 27:840–845Google Scholar
Kisin ER, Murray AR, Keane MJ, Shi XC, Schwegler-Berry D, Gorelik O, Arepalli S, Castranova V, Wallace WE, Kagan VE, Shvedova AA (2007) Single-walled carbon nanotubes: geno- and cytotoxic effects in lung fibroblast V79 cells. J Toxicol Environ Health A 70(24):2071–2079. https://doi.org/10.1080/15287390701601251 CrossRefGoogle Scholar
Klavarioti M, Mantzavinos D, Kassinos D (2009) Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ Int 35(2):402–417. https://doi.org/10.1016/j.envint.2008.07.009 CrossRefGoogle Scholar
Kwon JY, Kim HL, Lee JY, Ju YH, Kim JS, Kang SH, Kim YR, Lee JK, Jeong J, Kim MK, Maeng EH, Seo YR (2014) Undetactable levels of genotoxicity of SiO2 nanoparticles in in vitro and in vivo tests. Int J Nanomed 9(Suppl 2):173–1781. https://doi.org/10.2147/ijn.s57933 Google Scholar
Lajmanovich RC, Cabagna M, Peltzer PM, Stringhini GA, Attademo AM (2005) Micronucleus induction in erythrocytes of the Hyla pulchella tadpoles (Amphibia: Hylidae) exposed to insecticide endosulfan. Mutat Res 10:67–72. https://doi.org/10.1016/j.mrgentox.2005.08.001 CrossRefGoogle Scholar
Lajmanovich RC, Cabagna Zenklusen M, Attademo AM, Junges CM, Peltzer PM, Bassó A, Lorenzatti E (2014) Induction of micronuclei and nuclear abnormalities in common toad tadpoles (Rhinella arenarum) treated with Liberty® and glufosinate-ammonium. Mutat Res Genet Toxicol Environ Mutagen 15(769):7–12. https://doi.org/10.1016/j.mrgentox.2014.04.009 CrossRefGoogle Scholar
Landsiedel R, Kapp MD, Schulz M, Wiench K, Oesch F (2009) Genotoxicity investigations on nanomaterials: methods, preparation and characterization of test material, potential artifacts and limitations—many questions, some answers. Mutat Res 681(2–3):241–258. https://doi.org/10.1016/j.mrrev.2008.10.002 CrossRefGoogle Scholar
Li L, Stoiber M, Wimmer A, Xu Z, Lindenblatt C, Helmreich B, Schuster M (2016) To what extent can full-scale wastewater treatment plant effluent influence the occurrence of silver-based nanoparticles in surface waters? Environ Sci Technol 50:6327–6333. https://doi.org/10.1021/acs.est.6b00694 CrossRefGoogle Scholar
Lindsey ME, Meyer M, Thurman EM (2001) Analysis of trace levels of sulfonamide and tetracycline antimicrobials in groundwater and surface water using solid-phase extraction and liquid chromatography/mass spectrometry. Anal Chem 73(19):4640–4646. https://doi.org/10.1021/ac010514w CrossRefGoogle Scholar
Mahdi KNM, Commelin M, Peters RJB, Baartman JEM, Ritsema C, Geissen V (2017) Transport of silver nanoparticles by runoff and erosion—a flume experiment. Sci Total Environ 601–602:1418–1426. https://doi.org/10.1016/j.scitotenv.2017.06.020 CrossRefGoogle Scholar
Margolin BH, Collings BJ, Mason JM (1983) Statistical analysis and sample- size determinations for mutagenicity experiments with binomial responses. Environ Mutagen 5:705–716. https://doi.org/10.1002/em.2860050509 CrossRefGoogle Scholar
Martinuzzi C, Peltzer PM, Attademo AM, Junges CM, Lajmanovich RC (2016) Albinism in larvae of the Chacoan frog Leptodactylus chaquensis (Anura, Leptodactylidae) from an urban lake from Argentina. Cuadernos de Herpetología 30(2):69–73Google Scholar
McConnell L, Sparling DW (2010) Emerging contaminants and their potential effects on amphibians and reptiles. In: Sparling DW, Linder G, Bishop C, Krest S (eds) Ecotoxicology of amphibians and reptiles, 2nd edn. SETAC Press, Pensacola, pp 498–513Google Scholar
Melvin SD (2016) Oxidative stress, energy storage, and swimming performance of Limnodynastes peronii tadpoles exposed to a sub-lethal pharmaceutical mixture throughout development. Chemosphere 150:790–797. https://doi.org/10.1016/j.chemosphere.2015.09.034 CrossRefGoogle Scholar
Munteanu MC, Radu M, Hermenean A, Sima C, Dinu D, Costache M, Grigoriu C, Dinischiotu A (2010) Antioxidative response induced by SiO2 nanoparticles in MRC-5 cell line. Rom Biotech Lett 5(1):5000–5007Google Scholar
Napierska D, Thomassen LCJ, Lison D, Martens JA, Hoet PH (2010) The nanosilica hazard: another variable entity. Fibre Toxicol, Part. https://doi.org/10.1186/1743-8977-7-39 Google Scholar
Nations S, Long M, Wages M, Maul JD, Theodorakis CW, Cobb GP (2015) Subchronic and chronic developmental effects of copper oxide (CuO) nanoparticles on Xenopus laevis. Chemosphere 135:166–174. https://doi.org/10.1016/j.chemosphere.2015.03.078 CrossRefGoogle Scholar
Nishimoria H, Kondoha M, Isodaa K, Tsunodabc S, Tsutsumibcd Y, Yagia Y (2009) Silica nanoparticles as hepatotoxicants. Eur J Pharm Biopharm 72(3):496–501. https://doi.org/10.1016/j.ejpb.2009.02.005 CrossRefGoogle Scholar
Oruç EO, Sevgiler Y, Uner N (2004) Tissue-specific oxidative stress responses in fish exposed to 2,4-D and azinphosmethyl. Comp Biochem Physiol C 137(1):43–51. https://doi.org/10.1016/j.cca.2003.11.006 CrossRefGoogle Scholar
Ostroumov SA, Kotelevtsev SV (2011) Toxicology of nanomaterials and environment. Ecologica 18: 3–10 Google Scholar
Pacheco M, Santos MA (1997) Induction of EROD activity and genotoxic effects by polycyclic aromatic hydrocarbons and resin acids on the juvenile eell (Anguilla anguilla L.). Ecotoxicol Environ Saf 38:252–259. https://doi.org/10.1006/eesa.1997.1585 CrossRefGoogle Scholar
Peltzer PM, Lajmanovich RC, Attademo AM, Junges CM, Teglia CM, Martinuzzi C, Curi L, Culzoni MJ, Goicoechea HC (2017) Ecotoxicity of veterinary enrofloxacin and ciprofloxacin antibiotics on anuran amphibian larvae. Environ Toxicol Pharmacol 51:114–123. https://doi.org/10.1016/j.etap.2017.01.021 CrossRefGoogle Scholar
Persoone G, Marsalek B, Blinova I, Törökne A, Zarina D, Manusadzianas L, Nalecz-Jawecki G, Tofan L, Stepanova N, Tothova L, Kolar B (2003) A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ Toxicol 18(6):395–402. https://doi.org/10.1002/tox.10141 CrossRefGoogle Scholar
Petrache Voicu SN, Dinu D, Sima C, Hermenean A, Ardelean A, Codrici E, Stan MS, Zărnescu O, Dinischiotu A (2015) Silica nanoparticles induce oxidative stress and autophagy but not apoptosis in the MRC-5 cell line. Int J Mol Sci 16(12):29398–29416. https://doi.org/10.3390/ijms161226171 CrossRefGoogle Scholar
Phan VN, Gomes V, Passos MJACR, Ussami KA, Campos DYF, Rocha AJS, Pereira BA (2007) Biomonitoring of the genotoxic potential (micronucleus and erythrocyte nuclear abnormalities assay) of the Admiralty Bay water surrounding the Brazilian Antarctic Research Station Comandante Ferraz King George Island. Polar Biol 30(2):209–217. https://doi.org/10.4322/apa.2015.020 CrossRefGoogle Scholar
Ray PC, Yu H, Fu PP (2009) Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Health C 27(1):1–35. https://doi.org/10.1080/10590500802708267 CrossRefGoogle Scholar
Ryu HJ, Seong NW, So BJ, Seo H, Kim JH, Hong JS, Park MK, Kim MS, Kim YR, Cho KB, Seo MY, Kim MK, Maeng EH, Son SW (2014) Evaluation of silica nanoparticle toxicity after topical exposure for 90 days. Int J Nanomed 9(Suppl 2):127–136. https://doi.org/10.2147/IJN.S57929 Google Scholar
Sajid M, Ilyas M, Basheer C, Tariq M, Daud M, Baig N, Shehzad F (2015) Impact of nanoparticles on human and environment: review of toxicity factors, exposures, control strategies, and future prospects. Environ Sci Pollut Res Int 22(6):4122–4143. https://doi.org/10.1007/s11356-014-3994-1 CrossRefGoogle Scholar
Salvaterra T, Alves MG, Domingues I, Pereira R, Rasteiro MG, Carvalho RA, Soares AMVM, Lopes I (2013) Biochemical and metabolic effects of a short-term exposure to nanoparticles of titanium silicate in tadpoles of Pelophylax perezi (Seoane). Aquat Toxicol 128–129:190–192. https://doi.org/10.1016/j.aquatox.2012.12.014 CrossRefGoogle Scholar
Sauvé S, Desrosiers M (2014) A review of what is an emerging contaminant. Chem Cent J 8:15. https://doi.org/10.1186/1752-153X-8-15 CrossRefGoogle Scholar
Scherzad A, Meyer T, Kleinsasser N, Hackenberg S (2017) Molecular mechanisms of zinc oxide nanoparticle-induced genotoxicity. Materials (Basel) 10:1427. https://doi.org/10.3390/ma10121427 CrossRefGoogle Scholar
Sferratore A, Garnier J, Billen G, Conley DI, Pinault S (2006) Diffuse and point sources of silica in the Seine River watershed. Environ Sci Technol 40(21):6630–6635. https://doi.org/10.1021/es060710q CrossRefGoogle Scholar
Sgroi M, Roccaro P, Korshin GV, Vagliasindi FG (2017) Monitoring the behavior of emerging contaminants in wastewater-impacted rivers based on the use of fluorescence excitation emission matrixes (EEM). Environ Sci Technol 51(8):4306–4316. https://doi.org/10.1021/acs.est.6b05785 CrossRefGoogle Scholar
Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 18(22):1–9. https://doi.org/10.1088/0957-4484/18/22/225103 CrossRefGoogle Scholar
Thompson LB, Carfagno GLF, Andresen K, Sitton AJ, Bury T, Lee LL, Lerner KT, Fong PP (2017) Differential uptake of gold nanoparticles by 2 species of tadpole, the wood frog (Lithobates sylvaticus) and the bullfrog (Lithobates catesbeianus). Environ Toxicol Chem. https://doi.org/10.1002/etc.3909 (epub ahead of print) Google Scholar
Tiwari DK, Behari J (2009) Biocidal nature of treatment of Ag-nanoparticle and ultrasonic irradiation in Escherichia coli dh5. Adv Biol Res 3(3–4):89–95Google Scholar
U.S.EPA (U.S. Environmental Protection Agency) (1989) Short-term methods for estimating the chronic toxicity of effluents and receiving waters to fresh water organisms, Report EPA/600/4-89/001. Environmental Protection Agency, CincinnatiGoogle Scholar
Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF Jr, Rejeski D (2015) Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol 6:1769–1780. https://doi.org/10.3762/bjnano.6.181 CrossRefGoogle Scholar
Venturino A, Rosenbaum E, Caballero De Castro A, Anguiano O, Gauna L, Fonovich de Schroeder T, Pechen de D’Angelo AM (2003) Biomarkers of effect in toads and frogs. Biomarkers 8(3–4):167–186. https://doi.org/10.1080/1354700031000120116 CrossRefGoogle Scholar
Wang JJ, Sanderson BJS, Wang H (2007) Cytotoxicity and genotoxicity of ultrafine crystalline SiO2 particulate in cultured human lymphoblastoid cells. Environ Mol Mutagen 48:151–157. https://doi.org/10.1002/em.20287 CrossRefGoogle Scholar
Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, Roszek B, Bisschops J, Gosens I, van de Meent D, Dekkers S, de Jong WH, van Zijverden M, Sips AJAM, Geertsma RE (2009) Nano-silver: a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 3(2):109–138. https://doi.org/10.1080/17435390902725914 CrossRefGoogle Scholar
Ye RF, Yu XW, Yang SY, Yuan JL, Yang X (2013) Effects of silica dioxide nanoparticles on the embryonic development of zebrafish. Integr Ferroelectr 147:166–174. https://doi.org/10.1080/10584587.2013.792625 CrossRefGoogle Scholar
Zhang HY, Dunphy DR, Jiang XM, Meng H, Sun BB, Tarn D, Xue M, Wang X, Lin S, Ji Z, Li R, Garcia FL, Yang J, Kirk ML, Xia T, Zink JI, Nel A, Brinker CJ (2012) Processing pathway dependence of amorphous silica nanoparticle toxicity: colloidal vs pyrolytic. J Am Chem Soc 134(38):15790–15804. https://doi.org/10.1021/ja304907c CrossRefGoogle Scholar
Copyright information
© University of Tehran 2018
About this article
CrossMark
Cite this article as: Lajmanovich, R.C., Peltzer, P.M., Martinuzzi, C.S. et al. Int J Environ Res (2018). https://doi.org/10.1007/s41742-018-0089-8
DOI https://doi.org/10.1007/s41742-018-0089-8
Publisher Name Springer International Publishing
Print ISSN 1735-6865
Online ISSN 2008-2304
About this journal
Reprints and Permissions
Personalised recommendations
Download PDF
Actions
Download PDF
How to cite?
.RIS Papers Reference Manager RefWorks Zotero
.ENW EndNote
.BIB BibTeX JabRef Mendeley Share article
Table of contents
Article
Abstract
Introduction
Materials and Methods
Results
Discussion
Conclusions
Notes
References
Copyright information
About this article
Advertisement
**





